
-
 Beale to spearhead First Nations and Pasifika side against Lions
Beale to spearhead First Nations and Pasifika side against Lions
-
Wimbledon: England's garden Grand Slam

-
 Matcha: the Japanese tea taking over the world
Matcha: the Japanese tea taking over the world
-
Inter Milan, Monterrey join Dortmund in Club World Cup last 16

-
 Trail Blazers pick China's Yang in NBA draft first-round surprise
Trail Blazers pick China's Yang in NBA draft first-round surprise
-
Global matcha 'obsession' drinks Japan tea farms dry

-
 US judge sides with Meta in AI training copyright case
US judge sides with Meta in AI training copyright case
-
'Battle of Seattle' as Inter down nine-man River to advance

-
 China hosts Iranian, Russian defence ministers against backdrop of 'momentous change'
China hosts Iranian, Russian defence ministers against backdrop of 'momentous change'
-
Stocks down with eyes on Mideast, dollar hit by Trump Fed comment

-
 Syrian architect uses drone footage to help rebuild hometown
Syrian architect uses drone footage to help rebuild hometown
-
Verstappen hoping upgrades can boost title defence at Red Bull home race

-
 After 'Dune,' Denis Villeneuve to helm next James Bond film
After 'Dune,' Denis Villeneuve to helm next James Bond film
-
Thailand makes new proposal to restrict cannabis sales

-
 Ecuador's most-wanted gang leader 'Fito' captured
Ecuador's most-wanted gang leader 'Fito' captured
-
Tunisia U-turn on phosphate plant sparks anger in blighted city

-
 Trial of Sean 'Diddy' Combs heads into closing arguments
Trial of Sean 'Diddy' Combs heads into closing arguments
-
Wallabies release Reds pair Faessler and Paisami for Lions clash

-
 UN Charter: a founding document violated and ignored
UN Charter: a founding document violated and ignored
-
Vinicius, Mbappe have to defend: Real Madrid's Alonso

-
 US teen Cooper Flagg chosen by Mavericks with top pick in NBA draft
US teen Cooper Flagg chosen by Mavericks with top pick in NBA draft
-
Guardiola says City must be ready to 'suffer' in Orlando heat

-
 NBA studying uptick of Achilles injuries - Silver
NBA studying uptick of Achilles injuries - Silver
-
TransGlobal Assets Inc. (OTC:TMSH) Officially Launches DateGuard, Its Flagship Emotional Compatibility Dating App

-
 Pacquiao 'hungry' for comeback after four-year layoff
Pacquiao 'hungry' for comeback after four-year layoff
-
'Job done': Sundowns coach proud despite Club World Cup exit

-
 RFK Jr vaccine panel targets childhood vaccinations in first meeting
RFK Jr vaccine panel targets childhood vaccinations in first meeting
-
Tech giants' net zero goals verging on fantasy: researchers

-
 Australia quicks hit back after strong West Indies bowling effort
Australia quicks hit back after strong West Indies bowling effort
-
Dortmund through to Club World Cup last 16, Fluminense deny Sundowns

-
 Judge orders Trump admin to release billions in EV charging funds
Judge orders Trump admin to release billions in EV charging funds
-
Sale of NBA's $10 bn Lakers expected to close this year

-
 US Fed proposes easing key banking rule
US Fed proposes easing key banking rule
-
Nvidia hits fresh record while global stocks are mixed

-
 Elliott-inspired England to play Germany in Under-21 Euros final
Elliott-inspired England to play Germany in Under-21 Euros final
-
Gunmen kill 11 in crime-hit Mexican city

-
 Mbappe absent from Real Madrid squad for Salzburg Club World Cup clash
Mbappe absent from Real Madrid squad for Salzburg Club World Cup clash
-
Sainz opts out of race for FIA presidency

-
 Shamar Joseph rips through Australia top order in first Test
Shamar Joseph rips through Australia top order in first Test
-
Court rejects EDF complaint over Czech nuclear tender

-
 Mbappe returns to Real Madrid training at Club World Cup
Mbappe returns to Real Madrid training at Club World Cup
-
Kenya anniversary protests turn violent, 8 dead

-
 Elliott double fires England into Under-21 Euros final
Elliott double fires England into Under-21 Euros final
-
Trans campaigners descend on UK parliament to protest 'bathroom ban'

-
 New York mayoral vote floors Democratic establishment
New York mayoral vote floors Democratic establishment
-
Trump claims 'win' as NATO agrees massive spending hike

-
 EU probes Mars takeover of Pringles maker Kellanova
EU probes Mars takeover of Pringles maker Kellanova
-
Sidelined Zelensky still gets Trump face time at NATO summit

-
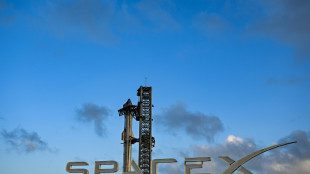 Mexico president threatens to sue over SpaceX rocket debris
Mexico president threatens to sue over SpaceX rocket debris
-
Amazon tycoon Bezos arrives in Venice for lavish wedding


Broad-Spectrum Antiviral Drug NV-387 Cleared for Phase II Clinical Trial Application by the National Ethics Committee of the Democratic Republic of Congo
SHELTON, CT / ACCESS Newswire / May 8, 2025 / NanoViricides, Inc. (NYSE Amer.:NNVC) (the "Company") today reported that it has received approval from the National Ethics Committee for Health (CNES) of the Ministry of Public Health (MSP), of the Democratic Republic of Congo (DRC). With this CNES approval, the proposed Phase II clinical trial to evaluate safety and effectiveness of NV-387 for the treatment of patients with MPox disease caused by hMPXV infection is cleared for further regulatory filing of a complete Clinical Trial Application ("CTA").
"We are now fully engaged in completing the detailed CTA for the Phase II human clinical trial of NV-387 for the treatment of MPOX disease (hMPXV infection) for submission to the DRC Regulatory Agency, namely MSP," said Anil R. Diwan, PhD, President and Executive Chairman of the Company.
The Company previously announced in January, 2025 that it has engaged a CRO for conducting a Phase II clinical trial to evaluate the safety and effectiveness of NV-387 for the treatment of MPox in the African Region.
Subsequently, the CRO has engaged the Medical Hospital at the University of Kinshasa as the clinical trial site (the "Site") for the Phase II clinical trial.
Thereafter, the Company, the CRO, and the Principal Investigator at the Site have developed a package of information comprising synopses of the clinical trial protocol, and required background information on the novel drug NV-387, including a draft summary report of the Phase I human clinical trial, prior non-clinical data, as well as summary of the animal model effectiveness and safety of NV-387 for the treatment of lethal MPox infection providing rationale for the use of the novel broad-spectrum antiviral drug NV-387 for the treatment of hMPXV viral infection and the MPox disease caused by the infection.
This summary package was submitted to the National Regulatory Agency's Ethics Committee CNES for a first review towards approval to proceed to the preparation and submission of the detailed CTA to the National Regulatory Agency, namely MSP of DRC.
The approval that we have now received from CNES clears the path for the perfected CTA filing to the MSP for approval and start of the clinical trial.
There is no drug available for the treatment of hMPXV infection that causes the MPox disease. A clinical trial of tecovirimat (TPOXX®, SIGA) failed to demonstrate any effectiveness over placebo, as per a NIH press release on August 15, 2024.
"NV-387, our broad-spectrum antiviral drug is poised to cause a revolution in treatment of viral diseases, just as antibiotics revolutionized the treatment of bacterial diseases," said Anil R. Diwan, Ph.D., adding "NV-387 is designed to mimic human cells to trap and destroy the virus. This single drug can target over 90-95% of human pathogenic viruses due to this biomimicry, which is reminiscent of the antibiotic penicillin that targets a large number of human pathogenic bacteria."
NV-387 was found to be highly effective in increasing survival in lethal animal models of influenza virus, surpassing existing drugs Tamiflu®, Rapivab® and Xofluza® by a large margin.
NV-387 led to a complete cure of lethal RSV lung infection in an animal model study. There is no approved drug for RSV treatment.
NV-387 was found to be highly effective in increasing survival in lethal animal models of Coronavirus infection (a stand-in model for SARS-CoV-2 infection), surpassing existing drug remdesivir by a large margin.
NV-387 was found to possess strong antiviral activity against an orthopoxvirus in an animal model that is considered an important model to establish potential effectiveness against MPox and Smallpox viruses, as all of these viruses belong to the same family of orthopoxviruses.
In fact, NV-387 effectiveness matched the effectiveness of the small chemical drug tecovirimat in two different models of infection, one was direct skin infection, and the other was a direct lung infection, by the virus.
Escape of virus from tecovirimat can occur by a single point mutation in a viral protein called VP-37.
Vaccines, antibodies, and small chemical drugs such as tecovirimat for MPox/Smallpox, or oseltamivir (Tamiflu®), baloxavir (Xofluza®) for Influenza are readily escaped by viruses simply by introduction of small changes that viruses undergo when they are faced with these challenges in the field.
In contrast, escape of virus from NV-387 is highly unlikely because no matter how much the virus changes in the field, it continues to use sulfated proteoglycans such as HSPG as "attachment receptor" in order to cause cell infection. NV-387 mimics the sulfated proteoglycan signature feature that the viruses require.
MPox disease, caused by the human MPox virus (hMPXV) has been causing a regional pandemic encompassing several countries in the WHO African Region that includs the Democratic Republic of Congo (DRC), Uganda, and other countries. It led to the WHO declaring a Public Health Emergency of International Concern ("PHEIC") on August 14, 2024. Since then, the PHEIC status declaration has been extended twice, most recently in March, 2025. The Mpox epidemic has continued to spread in the DRC, Uganda, and neighboring countries and the number of new weekly cases is still increasing in the WHO African Region.
NV-387 is a host-mimetic drug that "looks like a cell" to the virus, displaying numerous ligands that mimic the sulfated proteoglycan, enticing the virus to bind to and become engulfed by the NV-387 dynamic shape-shifting polymeric micelle.
Therefore development of NV-387, a broad-spectrum host-mimetic, direct-acting antiviral drug that the viruses cannot escape even as they change constantly, will be revolutionary once the drug undergoes regulatory development for approval for use in humans.
New viruses and existing viruses acquiring greater pathology and infectivity are bound to keep appearing in time. To combat such threats, we need to develop broad-spectrum drug arsenal that the viruses cannot escape. Vaccines and antibodies simply will not do, and their limitations have become clearly evident during the COVID-19 pandemic.
NanoViricides, Inc. (the "Company") (www.nanoviricides.com) is a clinical stage company that is creating special purpose nanomaterials for antiviral therapy. The Company's novel nanoviricide™ class of drug candidates and the nanoviricide™ technology are based on intellectual property, technology and proprietary know-how of TheraCour Pharma, Inc. The Company has a Memorandum of Understanding with TheraCour for the development of drugs based on these technologies for all antiviral infections. The MoU does not include cancer and similar diseases that may have viral origin but require different kinds of treatments.
The Company has obtained broad, exclusive, sub-licensable, field licenses to drugs developed in several licensed fields from TheraCour Pharma, Inc. The Company's business model is based on licensing technology from TheraCour Pharma Inc. for specific application verticals of specific viruses, as established at its foundation in 2005.
Our lead drug candidate is NV-387, a broad-spectrum antiviral drug that we plan to develop as a treatment of RSV, COVID, Long COVID, Influenza, and other respiratory viral infections, as well as MPOX/Smallpox infections. Our other advanced drug candidate is NV-HHV-1 for the treatment of Shingles. The Company cannot project an exact date for filing an IND for any of its drugs because of dependence on a number of external collaborators and consultants. The Company is currently focused on advancing NV-387 into Phase II human clinical trials.
NV-CoV-2 (API NV-387) is our nanoviricide drug candidate for COVID-19 that does not encapsulate remdesivir. NV-CoV-2-R is our other drug candidate for COVID-19 that is made up of NV-387 with remdesivir encapsulated within its polymeric micelles. The Company believes that since remdesivir is already US FDA approved, our drug candidate encapsulating remdesivir is likely to be an approvable drug, if safety is comparable. Remdesivir is developed by Gilead. The Company has developed both of its own drug candidates NV-CoV-2 and NV-CoV-2-R independently.
The Company is also developing drugs against a number of viral diseases including oral and genital Herpes, viral diseases of the eye including EKC and herpes keratitis, H1N1 swine flu, H5N1 bird flu, seasonal Influenza, HIV, Hepatitis C, Rabies, Dengue fever, and Ebola virus, among others. NanoViricides' platform technology and programs are based on the TheraCour® nanomedicine technology of TheraCour, which TheraCour licenses from AllExcel. NanoViricides holds a worldwide exclusive perpetual license to this technology for several drugs with specific targeting mechanisms in perpetuity for the treatment of the following human viral diseases: Human Immunodeficiency Virus (HIV/AIDS), Hepatitis B Virus (HBV), Hepatitis C Virus (HCV), Rabies, Herpes Simplex Virus (HSV-1 and HSV-2), Varicella-Zoster Virus (VZV), Influenza and Asian Bird Flu Virus, Dengue viruses, Japanese Encephalitis virus, West Nile Virus, Ebola/Marburg viruses, and certain Coronaviruses. The Company intends to obtain a license for RSV, Poxviruses, and/or Enteroviruses if the initial research is successful. As is customary, the Company must state the risk factor that the path to typical drug development of any pharmaceutical product is extremely lengthy and requires substantial capital. As with any drug development efforts by any company, there can be no assurance at this time that any of the Company's pharmaceutical candidates would show sufficient effectiveness and safety for human clinical development. Further, there can be no assurance at this time that successful results against coronavirus in our lab will lead to successful clinical trials or a successful pharmaceutical product.
This press release contains forward-looking statements that reflect the Company's current expectation regarding future events. Actual events could differ materially and substantially from those projected herein and depend on a number of factors. Certain statements in this release, and other written or oral statements made by NanoViricides, Inc. are "forward-looking statements" within the meaning of Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934. You should not place undue reliance on forward-looking statements since they involve known and unknown risks, uncertainties and other factors which are, in some cases, beyond the Company's control and which could, and likely will, materially affect actual results, levels of activity, performance or achievements. The Company assumes no obligation to publicly update or revise these forward-looking statements for any reason, or to update the reasons actual results could differ materially from those anticipated in these forward-looking statements, even if new information becomes available in the future. Important factors that could cause actual results to differ materially from the company's expectations include, but are not limited to, those factors that are disclosed under the heading "Risk Factors" and elsewhere in documents filed by the company from time to time with the United States Securities and Exchange Commission and other regulatory authorities. Although it is not possible to predict or identify all such factors, they may include the following: demonstration and proof of principle in preclinical trials that a nanoviricide is safe and effective; successful development of our product candidates; our ability to seek and obtain regulatory approvals, including with respect to the indications we are seeking; the successful commercialization of our product candidates; and market acceptance of our products.
The phrases "safety", "effectiveness" and equivalent phrases as used in this press release refer to research findings including clinical trials as the customary research usage and do not indicate evaluation of safety or effectiveness by the US FDA.
Where stated with an ® , the name is a registered trademark, which belongs to the owner of the trademark name.
FDA refers to US Food and Drug Administration. IND application refers to "Investigational New Drug" application. cGMP refers to current Good Manufacturing Practices. CMC refers to "Chemistry, Manufacture, and Controls". CHMP refers to the Committee for Medicinal Products for Human Use, which is the European Medicines Agency's (EMA) committee responsible for human medicines. API stands for "Active Pharmaceutical Ingredient". WHO is the World Health Organization. R&D refers to Research and Development.
Contact:
NanoViricides, Inc.
[email protected]
Public Relations Contact:
[email protected]
SOURCE: NanoViricides, Inc.
View the original press release on ACCESS Newswire
O.Johnson--AMWN