
-
 Inter Milan, Monterrey join Dortmund in Club World Cup last 16
Inter Milan, Monterrey join Dortmund in Club World Cup last 16
-
Trail Blazers pick China's Yang in NBA draft first-round surprise

-
 Global matcha 'obsession' drinks Japan tea farms dry
Global matcha 'obsession' drinks Japan tea farms dry
-
US judge sides with Meta in AI training copyright case

-
 'Battle of Seattle' as Inter down nine-man River to advance
'Battle of Seattle' as Inter down nine-man River to advance
-
China hosts Iranian, Russian defence ministers against backdrop of 'momentous change'

-
 Stocks down with eyes on Mideast, dollar hit by Trump Fed comment
Stocks down with eyes on Mideast, dollar hit by Trump Fed comment
-
Syrian architect uses drone footage to help rebuild hometown

-
 Verstappen hoping upgrades can boost title defence at Red Bull home race
Verstappen hoping upgrades can boost title defence at Red Bull home race
-
After 'Dune,' Denis Villeneuve to helm next James Bond film

-
 Thailand makes new proposal to restrict cannabis sales
Thailand makes new proposal to restrict cannabis sales
-
Ecuador's most-wanted gang leader 'Fito' captured

-
 Tunisia U-turn on phosphate plant sparks anger in blighted city
Tunisia U-turn on phosphate plant sparks anger in blighted city
-
Trial of Sean 'Diddy' Combs heads into closing arguments

-
 Wallabies release Reds pair Faessler and Paisami for Lions clash
Wallabies release Reds pair Faessler and Paisami for Lions clash
-
UN Charter: a founding document violated and ignored

-
 Vinicius, Mbappe have to defend: Real Madrid's Alonso
Vinicius, Mbappe have to defend: Real Madrid's Alonso
-
US teen Cooper Flagg chosen by Mavericks with top pick in NBA draft

-
 Guardiola says City must be ready to 'suffer' in Orlando heat
Guardiola says City must be ready to 'suffer' in Orlando heat
-
NBA studying uptick of Achilles injuries - Silver

-
 TransGlobal Assets Inc. (OTC:TMSH) Officially Launches DateGuard, Its Flagship Emotional Compatibility Dating App
TransGlobal Assets Inc. (OTC:TMSH) Officially Launches DateGuard, Its Flagship Emotional Compatibility Dating App
-
Pacquiao 'hungry' for comeback after four-year layoff

-
 'Job done': Sundowns coach proud despite Club World Cup exit
'Job done': Sundowns coach proud despite Club World Cup exit
-
RFK Jr vaccine panel targets childhood vaccinations in first meeting

-
 Tech giants' net zero goals verging on fantasy: researchers
Tech giants' net zero goals verging on fantasy: researchers
-
Australia quicks hit back after strong West Indies bowling effort

-
 Dortmund through to Club World Cup last 16, Fluminense deny Sundowns
Dortmund through to Club World Cup last 16, Fluminense deny Sundowns
-
Judge orders Trump admin to release billions in EV charging funds

-
 Sale of NBA's $10 bn Lakers expected to close this year
Sale of NBA's $10 bn Lakers expected to close this year
-
US Fed proposes easing key banking rule

-
 Nvidia hits fresh record while global stocks are mixed
Nvidia hits fresh record while global stocks are mixed
-
Elliott-inspired England to play Germany in Under-21 Euros final

-
 Gunmen kill 11 in crime-hit Mexican city
Gunmen kill 11 in crime-hit Mexican city
-
Mbappe absent from Real Madrid squad for Salzburg Club World Cup clash

-
 Sainz opts out of race for FIA presidency
Sainz opts out of race for FIA presidency
-
Shamar Joseph rips through Australia top order in first Test

-
 Court rejects EDF complaint over Czech nuclear tender
Court rejects EDF complaint over Czech nuclear tender
-
Mbappe returns to Real Madrid training at Club World Cup

-
 Kenya anniversary protests turn violent, 8 dead
Kenya anniversary protests turn violent, 8 dead
-
Elliott double fires England into Under-21 Euros final

-
 Trans campaigners descend on UK parliament to protest 'bathroom ban'
Trans campaigners descend on UK parliament to protest 'bathroom ban'
-
New York mayoral vote floors Democratic establishment

-
 Trump claims 'win' as NATO agrees massive spending hike
Trump claims 'win' as NATO agrees massive spending hike
-
EU probes Mars takeover of Pringles maker Kellanova

-
 Sidelined Zelensky still gets Trump face time at NATO summit
Sidelined Zelensky still gets Trump face time at NATO summit
-
Mexico president threatens to sue over SpaceX rocket debris
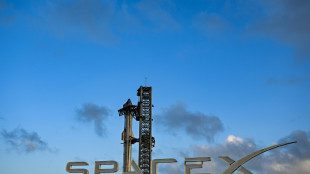
-
 Amazon tycoon Bezos arrives in Venice for lavish wedding
Amazon tycoon Bezos arrives in Venice for lavish wedding
-
Shamar Joseph gives West Indies strong start against Australia

-
 Raducanu's Wimbledon build-up hit by Eastbourne exit
Raducanu's Wimbledon build-up hit by Eastbourne exit
-
RFK Jr.'s vaccine panel opens amid backlash over fabricated study


Aptevo Therapeutics Provides Program Update for Bispecific APVO711, a PD-L1 X CD40 that Combines a Checkpoint Inhibitor and Immune Activation
As lead candidate mipletamig continues to outperform efficacy and safety benchmarks in AML trials, APVO711 exemplifies emerging innovation from Aptevo's proprietary ADAPTIR® platform
In preclinical studies, APVO711 demonstrates dual anti-cancer functionality with broad solid tumor potential and developability
SEATTLE, WASHINGTON / ACCESS Newswire / May 8, 2025 / Aptevo Therapeutics Inc. (Nasdaq:APVO), a clinical-stage biotechnology company focused on developing novel immune-oncology therapeutics based on its proprietary ADAPTIR® and ADAPTIR-FLEX® platform technologies, today provided a program update for APVO711, a dual mechanism bispecific utilizing the PD-L1 arm to block the PD-1/PD-L1 pathway and the CD40 arm to enhance T cell priming through activation of the stimulatory receptor CD40 on antigen presenting cells. This update highlights the depth and scientific versatility of Aptevo's existing pipeline and reinforces the Company's ability to advance differentiated candidates for solid tumors with significant unmet need. Currently in preclinical development, APVO711 reflects the potential of Aptevo's platform-based strategy to generate bispecific therapeutics with novel mechanisms and broad clinical relevance.
"With APVO711, we're advancing an innovative dual-mechanism approach to immunotherapy-one that not only boosts the immune system through PD-L1 blockade but also facilitates T-cell priming via CD40 activation. This is important in the development of new therapeutics for cancers that have proven resistant to standard checkpoint therapies. We believe APVO711 has the potential to unlock deeper, more sustained responses, and ultimately expand the reach of immunotherapy to patients who need it most," said Marvin White, President and CEO of Aptevo.
About APVO711
Novel Mechanism: APVO711 is a bispecific antibody targeting PD-L1 × CD40-combining checkpoint inhibition with immune activation in a single molecule.
Designed for Potency: Unlike traditional checkpoint inhibitors, APVO711 simultaneously blocks immune suppression and primes antigen presenting cells to trigger robust T-cell responses.
Targeting the Unmet Need: Developed to address a wide variety of tumor types and augment the anti-tumor responses achieved with PD-1/PD-L1 blockade alone.
Pipeline Synergy: Represents a key part of Aptevo's novel immuno-oncology platform, with strong potential for combination strategies across solid tumors.
Momentum Building: Preclinical studies demonstrate dual anti-cancer functionality.
"APVO711 represents a novel immunotherapy approach - by targeting both PD-L1 and CD40, this bispecific candidate is designed to block tumor-driven immune suppression while simultaneously priming T cells for a stronger, more durable anti-tumor response. In a landscape where many patients fail to respond to checkpoint inhibitors alone, APVO711 offers a rational strategy to expand the benefits of immunotherapy to a broader range of cancers and patients," said Michelle H. Nelson, Ph.D., Director of Immunobiology at Aptevo Therapeutics.
About Aptevo Therapeutics
Aptevo Therapeutics Inc. (Nasdaq: APVO) is a clinical-stage biotechnology company focused on developing novel bispecific immunotherapies for the treatment of cancer. The Company has two clinical candidates. Mipletamig is currently being evaluated in RAINIER, a two-part Phase 1b/2 trial for the treatment of frontline acute myeloid leukemia in combination with standard-of-care venetoclax + azacitidine. Mipletamig has received orphan drug designation ("orphan status") for AML according to the Orphan Drug Act. ALG.APV-527, a bispecific conditional 4-1BB agonist, only active upon simultaneous binding to 4-1BB and 5T4, is being co-developed with Alligator Bioscience and is being evaluated in a Phase 1 clinical trial for the treatment of multiple solid tumor types likely to express 5T4. The Company has three pre-clinical candidates with different mechanisms of action designed to target a range of solid tumors. All pipeline candidates were created from two proprietary platforms, ADAPTIR®and ADAPTIR-FLEX®. The Aptevo mission is to improve treatment outcomes and transform the lives of cancer patients. For more information, please visit www.aptevotherapeutics.com.
Safe Harbor Statement
This press release includes forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. All statements, other than statements of historical fact, including, without limitation, Aptevo's expectations about the activity, efficacy, safety, tolerability and durability of its therapeutic candidates and potential use of any such candidates, including in combination with other drugs, as therapeutics for treatment of disease, its expectations regarding the effectiveness of its ADAPTIR and ADAPTIR-FLEX platforms, whether pre-clinical studies of APVO711 will show the desired anti-tumor efficacy, mechanism of action and safety profile and whether APVO711 will function with new mechanisms of action compared to our previous candidates and synergistically induce a biological response, whether APVO711 will demonstrate the ability to fight a range of solid malignancies, statements related to the progress of Aptevo's clinical programs, including statements related to anticipated clinical and regulatory milestones, whether further study of mipletamig in a Phase 1b dose optimization trial focusing on multiple doses of mipletamig in combination with venetoclax + azacitidine on a targeted patient population will continue to show clinical benefit, whether Aptevo's final trial results will vary from its earlier assessment, the possibility and timing of interim data readouts for ALG.APV-527, statements related to Aptevo's ability to generate stockholder value, whether Aptevo will continue to have momentum in its business in the future, and any other statements containing the words "may," "continue to," "believes," "knows," "expects," "optimism," "potential," "designed," "promising," "plans," "will" and similar expressions are intended to identify forward-looking statements. These forward-looking statements are based on Aptevo's current intentions, beliefs, and expectations regarding future events. Aptevo cannot guarantee that any forward-looking statement will be accurate. Investors should realize that if underlying assumptions prove inaccurate or unknown risks or uncertainties materialize, actual results could differ materially from Aptevo's expectations. Investors are, therefore, cautioned not to place undue reliance on any forward-looking statement.
There are several important factors that could cause Aptevo's actual results to differ materially from those indicated by such forward-looking statements, including a deterioration in Aptevo's business or prospects; further assessment of preliminary or interim data or different results from later clinical trials; adverse events and unanticipated problems, adverse developments in clinical development, including unexpected safety issues observed during a clinical trial; and changes in regulatory, social, macroeconomic and political conditions. For instance, actual results may differ materially from those indicated by such forward-looking statements as a result of various important factors, including the uncertainties inherent in the results of preliminary or interim data and preclinical studies being predictive of the results of later-stage clinical trials, initiation, enrollment and maintenance of patients, and the completion of clinical trials, the availability and timing of data from ongoing clinical trials, the trial design includes combination therapies that may make it difficult to accurately ascertain the benefits of mipletamig, expectations for the timing and steps required in the regulatory review process, expectations for regulatory approvals, the impact of competitive products, our ability to enter into agreements with strategic partners or raise funds on acceptable terms or at all and other matters that could affect the availability or commercial potential of Aptevo's product candidates, business or economic disruptions due to catastrophes or other events, including natural disasters or public health crises such as the coronavirus (referred to as COVID-19), geopolitical risks, including the current war between Russia and Ukraine and the war between Israel and Hamas, and macroeconomic conditions such as economic uncertainty, rising inflation and interest rates, continued market volatility and decreased consumer confidence. These risks are not exhaustive, Aptevo faces known and unknown risks. Additional risks and factors that may affect results are set forth in Aptevo's filings with the Securities and Exchange Commission, including its Annual Report on Form 10-K for the fiscal year ended December 31, 2024, and its subsequent reports on Form 10-Q and current reports on Form 8-K. The foregoing sets forth many, but not all, of the factors that could cause actual results to differ from Aptevo's expectations in any forward-looking statement. Any forward-looking statement speaks only as of the date of this press release, and, except as required by law, Aptevo does not assume any obligation to update any forward-looking statement to reflect new information, events, or circumstances.
CONTACT:
Miriam Weber Miller
Head, Investor Relations & Corporate Communications
Aptevo Therapeutics
Email: [email protected] or [email protected]
Phone: 206-859-6628
SOURCE: Aptevo Therapeutics
View the original press release on ACCESS Newswire
M.Thompson--AMWN