
-
 'Job done': Sundowns coach proud despite Club World Cup exit
'Job done': Sundowns coach proud despite Club World Cup exit
-
RFK Jr vaccine panel targets childhood vaccinations in first meeting

-
 Tech giants' net zero goals verging on fantasy: researchers
Tech giants' net zero goals verging on fantasy: researchers
-
Australia quicks hit back after strong West Indies bowling effort

-
 Dortmund through to Club World Cup last 16, Fluminense deny Sundowns
Dortmund through to Club World Cup last 16, Fluminense deny Sundowns
-
Judge orders Trump admin to release billions in EV charging funds

-
 Sale of NBA's $10 bn Lakers expected to close this year
Sale of NBA's $10 bn Lakers expected to close this year
-
US Fed proposes easing key banking rule

-
 Nvidia hits fresh record while global stocks are mixed
Nvidia hits fresh record while global stocks are mixed
-
Elliott-inspired England to play Germany in Under-21 Euros final

-
 Gunmen kill 11 in crime-hit Mexican city
Gunmen kill 11 in crime-hit Mexican city
-
Mbappe absent from Real Madrid squad for Salzburg Club World Cup clash

-
 Sainz opts out of race for FIA presidency
Sainz opts out of race for FIA presidency
-
Shamar Joseph rips through Australia top order in first Test

-
 Court rejects EDF complaint over Czech nuclear tender
Court rejects EDF complaint over Czech nuclear tender
-
Mbappe returns to Real Madrid training at Club World Cup

-
 Kenya anniversary protests turn violent, 8 dead
Kenya anniversary protests turn violent, 8 dead
-
Elliott double fires England into Under-21 Euros final

-
 Trans campaigners descend on UK parliament to protest 'bathroom ban'
Trans campaigners descend on UK parliament to protest 'bathroom ban'
-
New York mayoral vote floors Democratic establishment

-
 Trump claims 'win' as NATO agrees massive spending hike
Trump claims 'win' as NATO agrees massive spending hike
-
EU probes Mars takeover of Pringles maker Kellanova

-
 Sidelined Zelensky still gets Trump face time at NATO summit
Sidelined Zelensky still gets Trump face time at NATO summit
-
Mexico president threatens to sue over SpaceX rocket debris
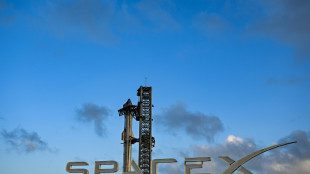
-
 Amazon tycoon Bezos arrives in Venice for lavish wedding
Amazon tycoon Bezos arrives in Venice for lavish wedding
-
Shamar Joseph gives West Indies strong start against Australia

-
 Raducanu's Wimbledon build-up hit by Eastbourne exit
Raducanu's Wimbledon build-up hit by Eastbourne exit
-
RFK Jr.'s vaccine panel opens amid backlash over fabricated study

-
 'You try not to bump into things:' blind sailing in Rio
'You try not to bump into things:' blind sailing in Rio
-
Trump says 'three or four' candidates in mind for Fed chief

-
 Trump teases Iran talks next week, says nuclear programme set back 'decades'
Trump teases Iran talks next week, says nuclear programme set back 'decades'
-
Turkey tussles with Australia to host 2026 UN climate talks

-
 Bielle-Biarrey 'fit' for Top 14 final after suffering concussion
Bielle-Biarrey 'fit' for Top 14 final after suffering concussion
-
James Webb telescope discovers its first exoplanet

-
 Kenya's Kipyegon seeks history with four minute mile attempt
Kenya's Kipyegon seeks history with four minute mile attempt
-
Gunmen kill 10 in crime-hit Mexican city

-
 Olympic surfing venue battling erosion threat
Olympic surfing venue battling erosion threat
-
Relief, joy as Israel reopens after Iran war ceasefire

-
 Spain upholds fine against Rubiales for Hermoso forced kiss
Spain upholds fine against Rubiales for Hermoso forced kiss
-
Iran hangs three more accused of spying as fears grow for Swede

-
 Australia choose to bat first in first Test against West Indies
Australia choose to bat first in first Test against West Indies
-
Gambhir backs India bowlers to 'deliver' despite first Test misery

-
 Trump reassures allies as NATO agrees 'historic' spending hike
Trump reassures allies as NATO agrees 'historic' spending hike
-
England's Duckett says mindset change behind Test success

-
 Trump sees 'progress' on Gaza, raising hopes for ceasefire
Trump sees 'progress' on Gaza, raising hopes for ceasefire
-
UK's Glastonbury Festival opens gates amid Kneecap controversy

-
 Oil rebounds as markets track Iran-Israel ceasefire
Oil rebounds as markets track Iran-Israel ceasefire
-
Cable theft in north France disrupts Eurostar traffic

-
 Cambodians at quiet Thai border plead for peace
Cambodians at quiet Thai border plead for peace
-
Trump plays nice as NATO eyes 'historic' spending hike


Protagonist Announces Icotrokinra Phase 3 Data on Difficult-to-Treat Scalp and Genital Psoriasis and Preclinical Data on PN-881 Presented at the Society for Investigative Dermatology Annual Meeting
Data from icotrokinra ICONIC-TOTAL show 66% of patients with scalp psoriasis and 77% with genital psoriasis achieved site-specific clear or almost clear skin at Week 16
Icotrokinra continues to demonstrate a standout combination of significant skin clearance (IGA 0/1) and a favorable safety profile in a once daily pill
PN-881, a first-in-class oral peptide targeting the IL-17 pathway, potently and selectively binds IL-17A and -17F, blocking the three dimeric forms of the cytokine
NEWARK, CA / ACCESS Newswire / May 9, 2025 / Protagonist Therapeutics, Inc. ("Protagonist" or the "Company") announced new clinical data from the Phase 3 ICONIC-TOTAL a study investigating icotrokinra, the first-in-class oral peptide antagonist targeting IL-23 receptor and preclinical characterization of PN-881, the first-in-class oral peptide antagonist blocking the three dimeric forms of IL-17 (AA, AF and FF), were presented separately today at the 2025 Society for Investigative Dermatology (SID) Annual Meeting in San Diego, CA.
The ICONIC-TOTAL study, conducted by Protagonist's collaboration partner, Johnson & Johnson, evaluated adults and adolescents 12 years of age and older with body surface area as low as 1% and at least moderate plaque psoriasis (PsO) affecting high-impact skin sites.
Key findings from the high-impact skin sites cohort of the icotrokinra ICONIC-TOTAL study [1] :
At week 16, 57% of patients treated with once daily icotrokinra achieved the study's primary endpoint with an Investigator's Global Assessment (IGA) b score of 0/1 (clear or almost clear skin) and a ≥2-grade improvement from baseline at Week 16 compared to 6% of patients receiving placebo (P
66% of patients with scalp psoriasis achieved a scalp-specific Investigator's Global Assessment (ss-IGA) c score of 0/1 compared to 11% receiving placebo (P
77% of patients with genital psoriasis achieved a static Physician's Global Assessment of Genitalia (sPGA-G) d score of 0/1 compared to 21% receiving placebo (P
In the smaller subset of patients with hand/foot psoriasis, patients showed a numerically higher rate of skin clearance at Week 16 with 42% achieving a hand and/or foot Physician's Global Assessment (hf-PGA) e score of 0/1 compared to 26% receiving placebo.
Icotrokinra demonstrated a favorable safety profile. A similar proportion of patients experienced adverse events (50% and 42%) and serious adverse events (0.5% and 1.9%) in icotrokinra and placebo respectively through Week 16, with no new safety signals identified.
PN-881 represents Protagonist's next generation of oral peptides for psoriasis. Key takeaways from the pre-clinical characterization of the IL-17 oral peptide antagonist PN-881 [2] :
Exhibited nanomolar to picomolar in vitro potency comparable to bimekizumab and superior (70-fold) to secukinumab.
Showed metabolic stability in several matrices across several species, making it a suitable candidate for oral delivery.
Demonstrated PD-based target engagement in a mouse IL-17 challenge model after oral dosing
Demonstrated dose-dependent efficacy with significant reduction in skin thickness in a 5-day rat IL-23 induced skin inflammation model after oral dosing.
"High-impact skin sites affected by psoriasis can be very challenging to treat effectively and patients often experience unique challenges profoundly impacting their daily lives," said Dinesh V. Patel, Ph.D., President and Chief Executive Officer at Protagonist. "The data presented by our partner today demonstrate that icotrokinra continues to have the potential to transform the current treatment paradigm based on the impressive results achieved with a convenient targeted therapy in the form of a once-daily pill. Additionally, we are thrilled to share the first detailed characterization of our next drug candidate in the I&I space, PN-881, the first-in-class oral peptide antagonist targeting the three dimeric forms of IL-17, including AA, AF, and FF. We look forward to progressing our fully owned asset PN-881 in human clinical studies in Q4 2025."
Editor's notes:
ICONIC-TOTAL is a Phase 3 randomized controlled trial (RCT) evaluating the efficacy and safety of icotrokinra compared with placebo for the treatment of plaque PsO in 311 participants (icotrokinra=208; placebo=103) with at least moderate severity affecting special areas (e.g., scalp, genital and/or hands and feet) with overall IGA score of 0 or 1 with at least a 2-grade improvement as the primary endpoint.
The IGA is a five-point scale with a severity score ranging from 0 to 4, where 0 indicates clear, 1 is minimal, 2 is mild, 3 is moderate, and 4 indicates severe disease. [3]
The ss-IGA is a five-point scale where scalp lesions are assessed in terms of clinical signs of redness, thickness, and scaliness on a severity score ranging from 0 to 4, where 0 indicates absence of disease, 1 is very mild, 2 is mild, 3 is moderate and 4 indicates severe disease. [4]
The sPGA-G is a six-point scale used to evaluate the severity of genital psoriasis at a given time point ranging from 0 to 5, where 0 indicates clear, 1 is minimal, 2 is mild, 3 is moderate, 4 is severe and 5 indicates very severe disease. [5]
The Physician's Global Assessment of Psoriasis on the Hands and/or Feet (hf-PGA) assesses the severity of hand and foot psoriasis using a 5-point scale to score the plaques on the hands and feet as: clear (0), almost clear (1), mild (2), moderate (3) and severe (4). [6]
About the ICONIC Clinical Development Program
The pivotal Phase 3 ICONIC clinical development program of icotrokinra (in adult and adolescent individuals with moderate-to-severe plaque PsO) was initiated with two studies in Q4 2023 - ICONIC-LEAD and ICONIC-TOTAL - pursuant to the license and collaboration agreement between Protagonist Therapeutics, Inc. and Janssen Biotech, Inc., a Johnson & Johson company. The ICONIC program is being conducted by Johnson & Johnson.
ICONIC-LEAD (NCT06095115) is a randomized controlled trial (RCT) to evaluate the efficacy and safety of icotrokinra compared with placebo in participants with moderate-to-severe plaque PsO, with PASI 90 and IGA score of 0 or 1 with at least a 2-grade improvement as co-primary endpoints.
ICONIC-TOTAL (NCT06095102) is a RCT to evaluate the efficacy and safety of icotrokinra compared with placebo for the treatment of PsO in participants with at least moderate severity affecting special areas (e.g., scalp, genital, and/or hands and feet) with overall IGA score of 0 or 1 with at least a 2-grade improvement as the primary endpoint.
Other Phase 3 studies in the development program include ICONIC-ADVANCE 1 (NCT06143878) and ICONIC-ADVANCE 2 (NCT06220604), which are evaluating the efficacy and safety of icotrokinra compared with both placebo and deucravacitinib in adults with moderate-to-severe plaque PsO. ICONIC-ASCEND will evaluate the efficacy and safety of icotrokinra compared with placebo and ustekinumab in participants with moderate-to-severe plaque psoriasis. ICONIC-PsA 1 (NCT06878404) and ICONIC-PsA 2 (NCT06807424) will evaluate the efficacy and safety of icotrokinra compared to placebo in participants with active psoriatic arthritis.
About Plaque Psoriasis
Plaque psoriasis (PsO) is a chronic immune-mediated disease resulting in overproduction of skin cells, which causes inflamed, scaly plaques that may be itchy or painful. It is estimated that 8 million Americans and more than 125 million people worldwide live with the disease. Nearly one-quarter of all people with plaque PsO have cases that are considered moderate to severe. On Caucasian skin, plaques typically appear as raised, red patches covered with a silvery white buildup of dead skin cells or scale. On skin of color, the plaques may appear darker and thicker and more of a purple, gray or dark brown color. Plaques can appear anywhere on the body, although they most often appear on the scalp, knees, elbows, and torso. Living with plaque PsO can be a challenge and impact life beyond a person's physical health, including emotional health, relationships, and handling the stressors of life. Psoriasis on highly visible areas of the body or sensitive skin, such as the scalp, hands, feet, and genitals, can have an increased negative impact on quality of life.
About Icotrokinra (JNJ-77242113, JNJ-2113)
Investigational icotrokinra is the first targeted oral peptide designed to selectively block the IL-23 receptor, which underpins the inflammatory response in moderate-to-severe plaque PsO, ulcerative colitis and offers potential in other IL-23-mediated diseases. Icotrokinra binds to the IL-23 receptor with single-digit picomolar affinity and demonstrated potent, selective inhibition of IL-23 signaling in human T cells. The license and collaboration agreement established between Protagonist Therapeutics, Inc. and Janssen Biotech, Inc., a Johnson & Johnson company, in 2017 enabled the companies to work together to discover and develop next-generation compounds that ultimately led to icotrokinra.
Icotrokinra was jointly discovered and is being developed pursuant to the license and collaboration agreement between Protagonist and Johnson & Johnson. Johnson & Johnson retains exclusive worldwide rights to develop icotrokinra in Phase 2 clinical trials and beyond, and to commercialize compounds derived from the research conducted pursuant to the agreement against a broad range of indications.
Icotrokinra is being studied in the pivotal Phase 3 ICONIC clinical development program in moderate-to-severe plaque psoriasis and active psoriatic arthritis and the Phase 2b ANTHEM-UC study in moderately to severely active ulcerative colitis.
About Protagonist
Protagonist Therapeutics is a discovery through late-stage development biopharmaceutical company. Two novel peptides, icotrokinra and rusfertide, derived from Protagonist's proprietary discovery platform are currently in advanced Phase 3 clinical development, with New Drug Application submissions to the FDA expected in 2025. Icotrokinra (JNJ-2113) is a first-in-class investigational targeted oral peptide that selectively blocks the Interleukin-23 receptor ("IL-23R") which is licensed to Janssen Biotech, Inc., a Johnson & Johnson company. Following icotrokinra's joint discovery by Protagonist and Johnson & Johnson scientists pursuant to the companies' IL-23R collaboration, Protagonist was primarily responsible for development of icotrokinra through Phase 1, with Johnson & Johnson assuming responsibility for development in Phase 2 and beyond. Rusfertide, a mimetic of the natural hormone hepcidin, is currently in Phase 3 development for the rare blood disorder polycythemia vera (PV). Rusfertide is being co-developed and will be co-commercialized with Takeda Pharmaceuticals pursuant to a worldwide collaboration and license agreement entered into in 2024 under which the Company remains primarily responsible for development through NDA filing. The Company also has a number of pre-clinical stage oral drug discovery programs addressing clinically and commercially validated targets, including the IL-17 oral peptide antagonist PN-881, an oral hepcidin program, and an oral obesity program.
More information on Protagonist, its pipeline drug candidates and clinical studies can be found on the Company's website at www.protagonist-inc.com.
Cautionary Note on Forward-Looking Statements
This press release contains forward-looking statements for purposes of the safe harbor provisions of the Private Securities Litigation Reform Act of 1995. Forward-looking statements include statements regarding the potential benefits of icotrokinra and PN-881, and expectations regarding the icotrokinra and PN-881 development programs. In some cases, you can identify these statements by forward-looking words such as "anticipate," "believe," "may," "will," "expect," or the negative or plural of these words or similar expressions. Forward-looking statements are not guarantees of future performance and are subject to risks and uncertainties that could cause actual results and events to differ materially from those anticipated, including, but not limited to, our ability to develop and commercialize our product candidates, our ability to earn milestone payments under our collaboration agreements with Janssen and Takeda, our ability to use and expand our programs to build a pipeline of product candidates, our ability to obtain and maintain regulatory approval of our product candidates, our ability to operate in a competitive industry and compete successfully against competitors that have greater resources than we do, and our ability to obtain and adequately protect intellectual property rights for our product candidates. Additional information concerning these and other risk factors affecting our business can be found in our periodic filings with the Securities and Exchange Commission, including under the heading "Risk Factors" contained in our most recently filed periodic reports on Form 10-K and Form 10-Q filed with the Securities and Exchange Commission. Forward-looking statements are not guarantees of future performance, and our actual results of operations, financial condition and liquidity, and the development of the industry in which we operate, may differ materially from the forward-looking statements contained in this press release. Any forward-looking statements that we make in this press release speak only as of the date of this press release. We assume no obligation to update our forward-looking statements, whether as a result of new information, future events or otherwise, after the date of this press release.
Investor Relations Contact
Corey Davis, Ph.D.
LifeSci Advisors
+1 212 915 2577
[email protected]
Media Contact
Virginia Amann, Founder/CEO
+1 833 500 0061 ext 1
ENTENTE Network of Companies
[email protected]
[1] Gooderham, M.J. et al. Phase 3 results from an innovative trial design of treating plaque psoriasis involving difficult-to-treat, high-impact sites with icotrokinra, a targeted oral peptide that selectively inhibits the IL-23-receptor. Presented at the 2025 Society for Investigative Dermatology (Abstract #LB1142). May 2025.
[2] Halladay, J et al. PN-881: First-in-class oral peptide targeting the IL-17 pathway. Presented at the 2025 Society for Investigative Dermatology (Abstract #0003). May 2025.
[3] Simpson E, Bissonnette R, Eichenfield LF, et al. The validated Investigator Global Assessment for Atopic Dermatitis (vIGA-AD™): The development and reliability testing of a novel clinical outcome measurement instrument for the severity of atopic dermatitis [published online April 25, 2020]. J Am Acad Dermatol. doi: 10.1016/j.jaad.2020.04.104.
[4] Foley P, et al. Efficacy of Guselkumab Compared with Adalimumab and Placebo for Psoriasis in Specific Body Regions. JAMA Dermatol. 2018;154(6):676-683.
[5] Merola JF, Bleakman AP, Gottlieb AB, et al. The Static Physician's Global Assessment of Genitalia: a clinical outcome measure for the severity of genital psoriasis. J Drugs Dermatol. 2017;16(8):793-799.
[6] Goldblum O, et al. Validation of the physician's global assessment of psoriasis of the hands and/or feet as a clinical endpoint. J Am Acad Dermatol. 2013:68(4)Supplement1:AB218.
SOURCE: Protagonist Therapeutics
View the original press release on ACCESS Newswire
Ch.Havering--AMWN