
-
 Family mourns Mexican naval cadet killed in New York bridge crash
Family mourns Mexican naval cadet killed in New York bridge crash
-
Chanel reports 28% drop in full-year profit

-
 Man City unveil De Bruyne tribute as star prepares to say farewell
Man City unveil De Bruyne tribute as star prepares to say farewell
-
Ukrainians feel no closer to peace after Trump-Putin call

-
 European nations increase pressure on Israel to stop broad Gaza offensive
European nations increase pressure on Israel to stop broad Gaza offensive
-
McCullum urges England to show 'humility' after rocky spell

-
 Top-selling French rapper laid to rest after death aged 31
Top-selling French rapper laid to rest after death aged 31
-
European stocks close higher as Wall Street dips

-
 EU plans two-euro flat fee on small parcels from outside bloc
EU plans two-euro flat fee on small parcels from outside bloc
-
Chess great Carlsen held to draw by 143,000 players

-
 US to limit Covid boosters to over-65s or those at high risk
US to limit Covid boosters to over-65s or those at high risk
-
Del Toro holds Giro lead as Hoole wins rainy time trial

-
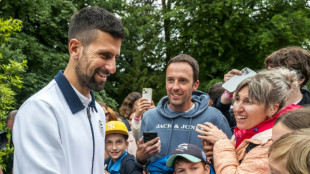 Djokovic says 'not in need of a coach' as French Open looms
Djokovic says 'not in need of a coach' as French Open looms
-
Rubio says Syria could be weeks away from 'full-scale civil war'

-
 Son dreaming of making history after 'unacceptable' Spurs season
Son dreaming of making history after 'unacceptable' Spurs season
-
Chelsea's Maresca fumes over Betis fixture change ahead of Conference League final

-
 'I'm not a clown': Spurs boss Postecoglou hits out ahead of Europa League final
'I'm not a clown': Spurs boss Postecoglou hits out ahead of Europa League final
-
Scarlett Johansson faces tough reviews in director debut

-
 Ahmedabad to host IPL final in revised schedule
Ahmedabad to host IPL final in revised schedule
-
Three dead as thunderstorms hit southeastern France

-
 Doucoure to leave Everton at end of season
Doucoure to leave Everton at end of season
-
Postecoglou fights to avoid sack as Spurs face Man Utd in Europa final

-
 Europa League final offers financial lifeline for Man Utd, Spurs
Europa League final offers financial lifeline for Man Utd, Spurs
-
Musk says will spend 'a lot less' on political campaigns

-
 'Kyiv should be ours': Russians boosted after Putin-Trump call
'Kyiv should be ours': Russians boosted after Putin-Trump call
-
Man Utd 'nowhere near good enough' admits Maguire

-
 Wall Street dips but European stocks rise
Wall Street dips but European stocks rise
-
S.Africans joke about Trump's claims ahead of White House visit

-
 Germany reports 40% jump in politically motivated crime
Germany reports 40% jump in politically motivated crime
-
Heatwave forces early school closures in Pakistan's largest province

-
 Iran's Panahi takes on Iran's jailers in Cannes comeback
Iran's Panahi takes on Iran's jailers in Cannes comeback
-
Adidas, Puma family feud to be turned into TV series

-
 Former England rugby star Brown to retire
Former England rugby star Brown to retire
-
Mother of jailed Egyptian-UK activist returns to full hunger strike

-
 Zelensky accuses Russia of buying time to stall peace talks
Zelensky accuses Russia of buying time to stall peace talks
-
Stocks rebound as China cuts rates

-
 Sherratt returns as Wales interim coach for Japan tour
Sherratt returns as Wales interim coach for Japan tour
-
Man Utd trio back training before Europa League final

-
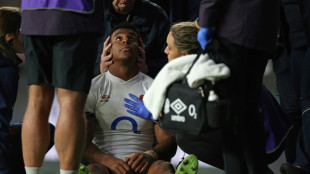 Feyi-Waboso included in England camp after injury lay-off
Feyi-Waboso included in England camp after injury lay-off
-
Indonesian gig drivers protest demanding lower app fees

-
 Leicester charged over alleged financial breaches
Leicester charged over alleged financial breaches
-
Dutch museum removes 'priceless' Benin Bronzes for return to Nigeria

-
 Gazan twins in Cannes warn 'nothing left' of homeland
Gazan twins in Cannes warn 'nothing left' of homeland
-
Dire sea level rise likely even in a 1.5C world: study

-
 Cannes film shines light on secret life of migrant maids
Cannes film shines light on secret life of migrant maids
-
WHO adopts landmark pandemic agreement

-
 Iran's Panahi pokes fun at Iran's jailers in Cannes comeback
Iran's Panahi pokes fun at Iran's jailers in Cannes comeback
-
Equities rebound to track Wall St up as China cuts rates

-
 Gaza rescuers say 44 killed as Israel steps up offensive
Gaza rescuers say 44 killed as Israel steps up offensive
-
'Sensation alert!': Chinese swimmer, 12, eyes world domination


Aspire Biopharma Holdings, Inc., Announces First Patient Dosed in Phase 1 Clinical Trial for its Lead Program, an Oral Transmucosal Fast-Acting High-Dose Aspirin Formulation
Trial marks key milestone in driving pipeline progress
Phase 1 trial scheduled for completion mid-June 2025
Topline data from High-Dose Aspirin Trail anticipated early in 3Q with the potential to support an accelerated approval, subject to FDA feedback
HUMACAO, PR and NEW YORK, NY / ACCESS Newswire / May 20, 2025 / Aspire Biopharma Holdings, Inc. (Nasdaq:ASBP) ("Aspire" or the "Company"), a developer of a multi-faceted patent-pending drug delivery technology, today announced that the first patient has been dosed in its Phase I single-center clinical trial in the United States designed to evaluate safety, pharmacokinetics and pharmacodynamics, of its lead therapeutic candidate, an oral transmucosal fast-acting high-dose aspirin formulation.
The objectives of this Phase 1 single dose clinical study are to evaluate the safety, pharmacokinetics and pharmacodynamics of Aspire's sublingual aspirin powder and granules when administered orally in healthy adult volunteers. The Phase 1 "crossover" clinical trial, which is being conducted in the United States, will compare the pharmacokinetic and pharmacodynamic characteristics of normal healthy adult volunteers administered a sublingual dose of 162.5 mg aspirin powder or granules with control healthy subjects given 162.5 mg oral aspirin (approximately two 81 mg aspirin tablets). The primary outcome measure will be plasma acetylsalicylic acid (ASA) concentration versus time data (pre-dose and up to 24 hours post dose.) This trial will also provide important data about TxB2 and its anti-coagulant bioavailability in the volunteers. For additional information on this trial please visit www.clinicaltrials.gov.
The Company expects to disclose topline data from its high-dose aspirin trial early in the third quarter and if successful, this trial has the potential to support accelerated approval, subject to FDA review.
"Dosing the first patient in our oral transmucosal fast-acting high-dose aspirin formulation study is an important milestone for Aspire as we continue the clinical development of our lead product candidate," said Kraig Higginson, Chief Executive Officer of Aspire. "We are proud that our aspirin formulation is one step closer to our goal of addressing several key unmet needs, and we are grateful to our patients for participating in this trial."
About the Aspire Targeted Oral Delivery Platform
Aspire's technology delivers a soluble, fast acting granular or powder form drug formulation which has been developed by using our patent-pending methodology, and "trade secret" process. The technologies new mechanism of action allows for rapid sublingual absorption and entry into the bloodstream. The benefits of "rapid absorption" are to provide nearly instant treatment impact and high dose absorption. The Company's patent-pending delivery system includes components specifically formulated to allow rapid sublingual absorption of drugs into the blood stream, thus by-passing the gastrointestinal tract, and potentially provide an improved treatment outcome.
About Aspire Biopharma, Inc.
Headquartered in Humacao, Puerto Rico, Aspire Biopharma has developed a disruptive technology through a Novel Soluble Formulation which addresses emergencies, drug efficacy, dosage management, and response time. For more information, please visit www.aspirebiolabs.com.
Safe Harbor Statement
Certain statements made in this communication are "forward-looking statements" within the meaning of the safe harbor provisions of the United States Private Securities Litigation Reform Act of 1995. Forward-looking statements may generally be identified by the use of words such as "estimate," "projects," "expects," "anticipates," "forecasts," "plans," "intends," "believes," "seeks," "may," "will," "would," "should," "future," "propose," "potential," "target," "goal," "objective," "outlook" and variations of these words or similar expressions (or the negative versions of such words or expressions) are intended to identify forward-looking statements. These forward-looking statements include, but are not limited to, statements regarding the financial position, business strategy and the plans and objectives of management for future operations. These statements are based on various assumptions, whether or not identified in this communication, and on the current expectations of Aspire's management and are not predictions of actual performance. These forward-looking statements are provided for illustrative purposes only and are not intended to serve as and must not be relied on by any investor as a guarantee, an assurance, a prediction or a definitive statement of fact or probability. These forward-looking statements are not guarantees of future performance, conditions or results, and involve a number of known and unknown risks, uncertainties, assumptions and other important factors, many of which are outside the control of the parties, that could cause actual results or outcomes to differ materially from those discussed in the forward-looking statements. The Company undertakes no obligation to update these statements for revisions or changes after the date of this release, except as required by law.
Aspire Biopharma Holdings, Inc.
Contact
TraDigital IR
Kevin McGrath
+1-646-418-7002
[email protected]
SOURCE: Aspire Biopharma Holdings, Inc.
View the original press release on ACCESS Newswire
P.M.Smith--AMWN