
-
 Family mourns Mexican naval cadet killed in New York bridge crash
Family mourns Mexican naval cadet killed in New York bridge crash
-
Chanel reports 28% drop in full-year profit

-
 Man City unveil De Bruyne tribute as star prepares to say farewell
Man City unveil De Bruyne tribute as star prepares to say farewell
-
Ukrainians feel no closer to peace after Trump-Putin call

-
 European nations increase pressure on Israel to stop broad Gaza offensive
European nations increase pressure on Israel to stop broad Gaza offensive
-
McCullum urges England to show 'humility' after rocky spell

-
 Top-selling French rapper laid to rest after death aged 31
Top-selling French rapper laid to rest after death aged 31
-
European stocks close higher as Wall Street dips

-
 EU plans two-euro flat fee on small parcels from outside bloc
EU plans two-euro flat fee on small parcels from outside bloc
-
Chess great Carlsen held to draw by 143,000 players

-
 US to limit Covid boosters to over-65s or those at high risk
US to limit Covid boosters to over-65s or those at high risk
-
Del Toro holds Giro lead as Hoole wins rainy time trial

-
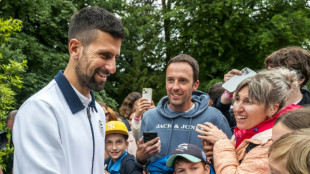 Djokovic says 'not in need of a coach' as French Open looms
Djokovic says 'not in need of a coach' as French Open looms
-
Rubio says Syria could be weeks away from 'full-scale civil war'

-
 Son dreaming of making history after 'unacceptable' Spurs season
Son dreaming of making history after 'unacceptable' Spurs season
-
Chelsea's Maresca fumes over Betis fixture change ahead of Conference League final

-
 'I'm not a clown': Spurs boss Postecoglou hits out ahead of Europa League final
'I'm not a clown': Spurs boss Postecoglou hits out ahead of Europa League final
-
Scarlett Johansson faces tough reviews in director debut

-
 Ahmedabad to host IPL final in revised schedule
Ahmedabad to host IPL final in revised schedule
-
Three dead as thunderstorms hit southeastern France

-
 Doucoure to leave Everton at end of season
Doucoure to leave Everton at end of season
-
Postecoglou fights to avoid sack as Spurs face Man Utd in Europa final

-
 Europa League final offers financial lifeline for Man Utd, Spurs
Europa League final offers financial lifeline for Man Utd, Spurs
-
Musk says will spend 'a lot less' on political campaigns

-
 'Kyiv should be ours': Russians boosted after Putin-Trump call
'Kyiv should be ours': Russians boosted after Putin-Trump call
-
Man Utd 'nowhere near good enough' admits Maguire

-
 Wall Street dips but European stocks rise
Wall Street dips but European stocks rise
-
S.Africans joke about Trump's claims ahead of White House visit

-
 Germany reports 40% jump in politically motivated crime
Germany reports 40% jump in politically motivated crime
-
Heatwave forces early school closures in Pakistan's largest province

-
 Iran's Panahi takes on Iran's jailers in Cannes comeback
Iran's Panahi takes on Iran's jailers in Cannes comeback
-
Adidas, Puma family feud to be turned into TV series

-
 Former England rugby star Brown to retire
Former England rugby star Brown to retire
-
Mother of jailed Egyptian-UK activist returns to full hunger strike

-
 Zelensky accuses Russia of buying time to stall peace talks
Zelensky accuses Russia of buying time to stall peace talks
-
Stocks rebound as China cuts rates

-
 Sherratt returns as Wales interim coach for Japan tour
Sherratt returns as Wales interim coach for Japan tour
-
Man Utd trio back training before Europa League final

-
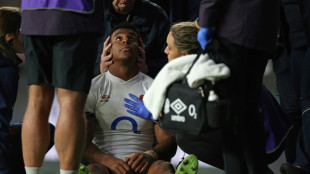 Feyi-Waboso included in England camp after injury lay-off
Feyi-Waboso included in England camp after injury lay-off
-
Indonesian gig drivers protest demanding lower app fees

-
 Leicester charged over alleged financial breaches
Leicester charged over alleged financial breaches
-
Dutch museum removes 'priceless' Benin Bronzes for return to Nigeria

-
 Gazan twins in Cannes warn 'nothing left' of homeland
Gazan twins in Cannes warn 'nothing left' of homeland
-
Dire sea level rise likely even in a 1.5C world: study

-
 Cannes film shines light on secret life of migrant maids
Cannes film shines light on secret life of migrant maids
-
WHO adopts landmark pandemic agreement

-
 Iran's Panahi pokes fun at Iran's jailers in Cannes comeback
Iran's Panahi pokes fun at Iran's jailers in Cannes comeback
-
Equities rebound to track Wall St up as China cuts rates

-
 Gaza rescuers say 44 killed as Israel steps up offensive
Gaza rescuers say 44 killed as Israel steps up offensive
-
'Sensation alert!': Chinese swimmer, 12, eyes world domination


First Patient Randomized in Jaguar Health’s Phase 2 Randomized, Double-Blind, Placebo-Controlled Clinical Trial of Crofelemer in Pediatric Patients with Microvillus Inclusion Disease (MVID)
As announced, initial proof-of-concept (POC) results from distinct ongoing investigator-initiated trial (IIT) in Abu Dhabi show crofelemer reduced total parenteral support in a pediatric MVID patient with intestinal failure by up to 27%; per the IIT protocol, patient was taken off crofelemer after 12 weeks of treatment for a period of 30 days, but was restarted on daily crofelemer treatment after just 8 days, as patient's symptoms were worsening
Additional POC IIT results expected throughout 2025 for MVID; results from company's MVID Phase 2 study expected with completion of treatment in H2 2026
Ground-breaking results in single-digit number of MVID patients could potentially lead to expedited approval in the EU through PRIME and to Breakthrough Therapy designation in the US
SAN FRANCISCO, CA / ACCESS Newswire / May 20, 2025 / Jaguar Health, Inc. (NASDAQ:JAGX) (Jaguar) family companies Napo Pharmaceuticals, Inc. (Napo) and Napo Therapeutics S.p.A. today announced that the first patient has been randomized in Napo's randomized, double-blind, placebo-controlled Phase 2 clinical trial to evaluate the safety and efficacy of the novel crofelemer oral powder for solution formulation in pediatric patients with intestinal failure due to the ultrarare disease MVID.
Patients with MVID suffer from devastating diarrhea and dehydration caused by this debilitating, lifelong condition. These pediatric patients often require lifelong and life-sustaining total parenteral nutrition (TPN) with additional supplemental intravenous (IV) fluids, termed total parenteral support, which is the standard of care for MVID, for up to 7 days a week greater than 50% of the time each day to provide adequate nutrient and electrolyte support. While crucial for MVID patients, TPN carries a significant risk of morbidity and mortality due to infections, metabolic complications, liver and/or kidney disease, and a risk of neurodevelopmental delay. There are currently no approved drug treatments for MVID.
As previously announced, and as presented last month at the 11th Annual ELITE PED-GI Congress in Abu Dhabi in the United Arab Emirates, the initial proof-of-concept results from the ongoing and independent proof-of-concept investigator-initiated trial (IIT) at Sheikh Khalifa Medical City (SKMC) showed that crofelemer reduced the required TPN and/or supplementary intravenous fluids - collectively referred to as parenteral support - in the first participating MVID patient by up to 27% at the end of 12-weeks of crofelemer treatment. In addition, this data showed that crofelemer reduced stool volume output and/or frequency of watery stools, and increased urine output - an indicator of improved nutrient oral absorption.
Per the protocol for the IIT at SKMC, the pediatric MVID patient was taken off crofelemer after 12 weeks of treatment for a period of 30 days. After just 8 days, the patient's parents requested reinitiation of crofelemer dosing, as the patient's symptoms were worsening - evidenced by increased stool output and decreased urine output. Hence, the patient was restarted on daily treatment with crofelemer.
"We're very happy that the first patient has been randomized in Napo's randomized, double-blind placebo-controlled Phase 2 clinical trial of crofelemer in pediatric MVID patients," said Lisa Conte, Jaguar's founder, president, and CEO. "This is another important milestone in development efforts for crofelemer for the treatment and management of intestinal failure related to this devastating ultrarare pediatric disease. For this study, randomization represents the key milestone of assigning a participating patient to either the crofelemer or placebo treatment group in a double-blind, placebo-controlled within-patient crossover study."
"Additional proof-of-concept results for MVID from the ongoing IIT of crofelemer at SKMC in pediatric patients with intestinal failure due to MVID and other disorders are expected throughout 2025," Conte said. "The results of Napo's randomized double-blind, placebo-controlled Phase 2 study of crofelemer in pediatric MVID patients are expected to be available in the second half of 2026. Because the IIT of crofelemer for MVID is an unblinded, open-label study, results from this IIT are expected to be available sooner."
Based on the initial proof-of-concept findings presented last month at the 11th Annual ELITE PED-GI Congress, crofelemer's novel antisecretory mechanism of action appears to have the potential to provide a novel therapeutic option to modify the disease progression associated with intestinal failure due to MVID in pediatric patients, as evidenced by the reductions in total parenteral support and potential to improve the quality of life of MVID patients.
"Based on preliminary communications with European Medicines Agency (EMA) regulatory members that focus on PRIME (Priority Medicines), a program that provides enhanced interactions and early dialogue for development of novel medicines targeting unmet medical needs, we are excited by the initial findings from the IIT being conducted at SKMC," said Conte. "We are not aware of any other intervention that has shown the ability to reduce the required administration of life-sustaining parenteral support including TPN, thus potentially improving nutrient absorption in patients with MVID. Given the ultrarare nature of MVID, even a small number of MVID patients showing benefit with crofelemer may allow Napo to explore pathways for expedited regulatory approval in the European Union. Crofelemer may qualify for participation in PRIME in the European Union for MVID and potentially in the U.S. Food and Drug Administration's (FDA) Breakthrough Therapy program for this ultrarare pediatric indication. Additionally, in accordance with the guidelines of specific EU countries, published data from clinical investigations in MVID could support reimbursed early patient access to crofelemer for this debilitating condition."
About Crofelemer
Crofelemer is a novel, oral plant-based prescription medicine purified from the red bark sap, also referred to as "dragon's blood," of the Croton lechleri tree in the Amazon Rainforest. Napo has established a sustainable harvesting program, under fair trade practices, for crofelemer to ensure a high degree of quality, ecological integrity, and support for indigenous communities.
About the Jaguar Health Family of Companies
Jaguar Health, Inc. (Jaguar) is a commercial stage pharmaceuticals company focused on developing novel proprietary prescription medicines sustainably derived from plants from rainforest areas for people and animals with gastrointestinal distress, specifically associated with overactive bowel, which includes symptoms such as chronic debilitating diarrhea, urgency, bowel incontinence, and cramping pain. Jaguar family company Napo Pharmaceuticals (Napo) focuses on developing and commercializing human prescription pharmaceuticals for essential supportive care and management of neglected gastrointestinal symptoms across multiple complicated disease states. Napo's crofelemer is FDA-approved under the brand name Mytesi® for the symptomatic relief of noninfectious diarrhea in adults with HIV/AIDS on antiretroviral therapy. Jaguar family company Napo Therapeutics is an Italian corporation Jaguar established in Milan, Italy in 2021 focused on expanding crofelemer access in Europe and specifically for orphan and/or rare diseases. Jaguar Animal Health is a Jaguar tradename. Magdalena Biosciences, a joint venture formed by Jaguar and Filament Health Corp. that emerged from Jaguar's Entheogen Therapeutics Initiative (ETI), is focused on developing novel prescription medicines derived from plants for mental health indications.
For more information about:
Jaguar Health, visit https://jaguar.health
Napo Pharmaceuticals, visit www.napopharma.com
Napo Therapeutics, visit napotherapeutics.com
Magdalena Biosciences, visit magdalenabiosciences.com
Visit the Make Cancer Less Shitty patient advocacy program on Bluesky, X, Facebook & Instagram
Forward-Looking Statements
Certain statements in this press release constitute "forward-looking statements." These include statements regarding Jaguar's expectation that additional POC results for MVID from the IIT in pediatric patients with intestinal failure due to MVID and other disorders may be available throughout 2025, Jaguar's expectation that results from the company's MVID Phase 2 study will be available with completion of treatment in H2 2026, Jaguar's expectation that crofelemer's novel antisecretory mechanism of action may have the potential to provide a novel therapeutic option to modify the disease progression associated with intestinal failure due to MVID in pediatric patients and the potential to improve the quality of life of MVID patients, Jaguar's expectation that, given the ultrarare nature of MVID, even a small number of MVID patients showing benefit with crofelemer may allow Napo to explore pathways for expedited regulatory approval in the Europe Union, Jaguar's expectation that crofelemer may qualify for participation in PRIME in the European Union for MVID and potentially in the FDA's Breakthrough Therapyprogram, and Jaguar's expectation that, in accordance with the guidelines of specific EU countries, published data from clinical investigations in MVID could support reimbursed early patient access to crofelemer for this debilitating condition. In some cases, you can identify forward-looking statements by terms such as "may," "will," "should," "expect," "plan," "aim," "anticipate," "could," "intend," "target," "project," "contemplate," "believe," "estimate," "predict," "potential" or "continue" or the negative of these terms or other similar expressions. The forward-looking statements in this release are only predictions. Jaguar has based these forward-looking statements largely on its current expectations and projections about future events. These forward-looking statements speak only as of the date of this release and are subject to several risks, uncertainties, and assumptions, some of which cannot be predicted or quantified and some of which are beyond Jaguar's control. Except as required by applicable law, Jaguar does not plan to publicly update or revise any forward-looking statements contained herein, whether as a result of any new information, future events, changed circumstances or otherwise.
Contact:
[email protected]
Jaguar-JAGX
SOURCE: Jaguar Health, Inc.
View the original press release on ACCESS Newswire
X.Karnes--AMWN