
-
 Trans campaigners descend on UK parliament to protest 'bathroom ban'
Trans campaigners descend on UK parliament to protest 'bathroom ban'
-
New York mayoral vote floors Democratic establishment

-
 Trump claims 'win' as NATO agrees massive spending hike
Trump claims 'win' as NATO agrees massive spending hike
-
EU probes Mars takeover of Pringles maker Kellanova

-
 Sidelined Zelensky still gets Trump face time at NATO summit
Sidelined Zelensky still gets Trump face time at NATO summit
-
Mexico president threatens to sue over SpaceX rocket debris
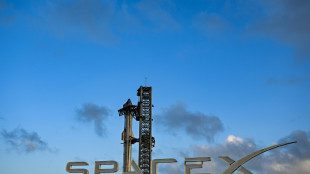
-
 Amazon tycoon Bezos arrives in Venice for lavish wedding
Amazon tycoon Bezos arrives in Venice for lavish wedding
-
Shamar Joseph gives West Indies strong start against Australia

-
 Raducanu's Wimbledon build-up hit by Eastbourne exit
Raducanu's Wimbledon build-up hit by Eastbourne exit
-
RFK Jr.'s vaccine panel opens amid backlash over fabricated study

-
 'You try not to bump into things:' blind sailing in Rio
'You try not to bump into things:' blind sailing in Rio
-
Trump says 'three or four' candidates in mind for Fed chief

-
 Trump teases Iran talks next week, says nuclear programme set back 'decades'
Trump teases Iran talks next week, says nuclear programme set back 'decades'
-
Turkey tussles with Australia to host 2026 UN climate talks

-
 Bielle-Biarrey 'fit' for Top 14 final after suffering concussion
Bielle-Biarrey 'fit' for Top 14 final after suffering concussion
-
James Webb telescope discovers its first exoplanet

-
 Kenya's Kipyegon seeks history with four minute mile attempt
Kenya's Kipyegon seeks history with four minute mile attempt
-
Gunmen kill 10 in crime-hit Mexican city

-
 Olympic surfing venue battling erosion threat
Olympic surfing venue battling erosion threat
-
Relief, joy as Israel reopens after Iran war ceasefire

-
 Spain upholds fine against Rubiales for Hermoso forced kiss
Spain upholds fine against Rubiales for Hermoso forced kiss
-
Iran hangs three more accused of spying as fears grow for Swede

-
 Australia choose to bat first in first Test against West Indies
Australia choose to bat first in first Test against West Indies
-
Gambhir backs India bowlers to 'deliver' despite first Test misery

-
 Trump reassures allies as NATO agrees 'historic' spending hike
Trump reassures allies as NATO agrees 'historic' spending hike
-
England's Duckett says mindset change behind Test success

-
 Trump sees 'progress' on Gaza, raising hopes for ceasefire
Trump sees 'progress' on Gaza, raising hopes for ceasefire
-
UK's Glastonbury Festival opens gates amid Kneecap controversy

-
 Oil rebounds as markets track Iran-Israel ceasefire
Oil rebounds as markets track Iran-Israel ceasefire
-
Cable theft in north France disrupts Eurostar traffic

-
 Cambodians at quiet Thai border plead for peace
Cambodians at quiet Thai border plead for peace
-
Trump plays nice as NATO eyes 'historic' spending hike

-
 Barcelona announce Camp Nou return for August 10
Barcelona announce Camp Nou return for August 10
-
Trump insists Iran nuclear programme set back 'decades'

-
 Armenia PM says foiled 'sinister' coup plot by senior cleric
Armenia PM says foiled 'sinister' coup plot by senior cleric
-
Turkey breathes easier as Iran-Israel truce eases fallout risk

-
 Tesla sales skid in Europe in May despite EV rebound
Tesla sales skid in Europe in May despite EV rebound
-
'Not Test class': Pundits tear into India after England chase 371

-
 Trump whirlwind tests NATO summit unity
Trump whirlwind tests NATO summit unity
-
Justice orders release of migrants deported to Costa Rica by Trump

-
 Vietnam tycoon will not face death penalty over $27 bn fraud: lawyer
Vietnam tycoon will not face death penalty over $27 bn fraud: lawyer
-
Vietnam abolishes death penalty for spying, anti-state activities

-
 Over 80,000 people flee severe flooding in southwest China
Over 80,000 people flee severe flooding in southwest China
-
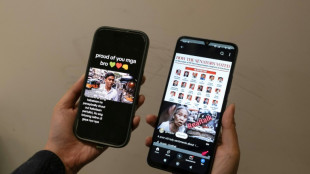
AI fakes duel over Sara Duterte impeachment in Philippines
-
 UK carbon emissions cut by half since 1990: experts
UK carbon emissions cut by half since 1990: experts
-
Delap off mark as Chelsea ease into Club World Cup last 16

-
 UK to reintroduce nuclear weapon-capable aircraft under NATO
UK to reintroduce nuclear weapon-capable aircraft under NATO
-
Upstart socialist stuns political veteran in NYC mayoral primary

-
 China's premier warns global trade tensions 'intensifying'
China's premier warns global trade tensions 'intensifying'
-
Chelsea through to Club World Cup knockouts, Benfica beat Bayern


iTolerance, Inc. Appoints Former FDA Senior Executive Wayne Pines to the Board of Directors
MIAMI, FL / ACCESS Newswire / June 25, 2025 / iTolerance, Inc. ("iTolerance" or the "Company"), an early-stage privately-held biotechnology company focused on the development of innovative regenerative medicines, today announced the appointment of Wayne Pines to the Company's Board of Directors. Mr. Pines has served as a member of the Company's Strategic Advisory Board since 2023.
"Wayne has been an integral part of our Strategic Advisory Board and has provided helpful insight as the Company continues to progress our pipeline forward. We are pleased to have him join the Board and believe his expertise will continue to be incredibly important for iTolerance," said Mitchell Robbins, Chairman of the iTolerance Board of Directors.
"We are thrilled to have Wayne join our Board of Directors. We believe this expansion of our Board is an excellent fit as we transition to a clinical-stage company. As stem cell-derived pancreatic islet products move closer to commercialization, iTOL-100, iTolerance's immunomodulator, has the potential to remove the need for life-long immunosuppression for these advanced therapies in Type 1 Diabetes. Wayne's extensive experience with the FDA will help us advance our pipeline and pursue our mission of developing transformative therapies for people living with diabetes and for doctors in search of better treatment options for patients," commented Anthony Japour, Chief Executive Officer of iTolerance.
Mr. Pines said: "iTolerance's platform technology using pancreatic islets continues to show promise as a potential treatment option for Type 1 Diabetes by eliminating the need for chronic systemic immunosuppression. This has the potential to be transformational to the Type 1 Diabetes community. I am honored by the opportunity to further support iTolerance as a member of the Board."

Mr. Pines serves as Senior Director and a member of the International Advisory Council at APCO in Washington, D.C. Mr. Pines is an international consultant on issues related to the Food and Drug Administration (FDA), including media, legislative, regulatory and marketing challenges, and other government agencies. He advises clients on government policies, navigating products through the FDA approval process, and promotional issues.
Mr. Pines served for ten years in senior positions at the FDA, including as Chief of Consumer Education and Information, Chief of Press Relations and Associate Commissioner for Public Affairs. In 2020, he served as a Senior Advisor on COVID-19 to the FDA commissioner. He has authored or edited 16 books about the FDA, including about the product approval process, FDA's regulation of medical communications, crisis management, and the history of the FDA. His latest book, published in 2022, is "How FDA Really Works: Insights from the Experts." He is widely published and quoted in the media about the FDA and health care issues and policies. He also is co-host of the podcast FDAWatch (www.fdawatch.net).
Mr. Pines was a Director and former Chairman of the Board of the MedStar Health Research Institute, which oversees research at ten hospitals in the Washington-Baltimore area. He is a Founder, Director and former President of the Alliance for a Stronger FDA, a coalition seeking more appropriated funding for FDA. He was a Co-Founder of the FDA Alumni Association; a member of the Public Health Service's first Task Force on AIDS Education; Executive Vice President of an international public relations agency; and Chairman of a health care market research firm. He also serves as a member of the Executive Committee of the Regional Board of the Anti-Defamation League.
About iTolerance, Inc.
iTolerance is a regenerative medicine company developing technologies to enable tissue, organoid or cell therapy without requiring life-long immunosuppression. Leveraging its proprietary biotechnology-derived Streptavidin-FasL fusion protein/biotin-PEG microgel (SA-FasL microgel) platform technology, iTOL-100, iTolerance is advancing a pipeline of programs using both allogenic cadaveric and stem cell-derived pancreatic islets to potentially cure Type 1 diabetes. Utilizing iTOL-100 to induce local immune tolerance, the Company is developing its lead indication as a potential cure for Type 1 Diabetes without the need for life-long immunosuppression. Additionally, the Company is developing iTOL-201 for treating liver failure by utilizing hepatocytes and iTOL-401 as a nanoparticle formulation for large organ transplants without the need for life-long immunosuppression. For more information, please visit itolerance.com.
Forward-Looking Statements
This press release contains "forward-looking statements" within the meaning of the "safe-harbor" provisions of the Private Securities Litigation Reform Act of 1995. When used herein, words such as "anticipate", "being", "will", "plan", "may", "continue", and similar expressions are intended to identify forward-looking statements. In addition, any statements or information that refer to expectations, beliefs, plans, projections, objectives, performance or other characterizations of future events or circumstances, including any underlying assumptions, are forward-looking.
All forward-looking statements are based upon the Company's current expectations and various assumptions. The Company believes there is a reasonable basis for its expectations and beliefs, but they are inherently uncertain. The Company may not realize its expectations, and its beliefs may not prove correct. Actual results could differ materially from those described or implied by such forward-looking statements as a result of various important factors, including, without limitation, anticipated levels of revenues, future national or regional economic and competitive conditions, and difficulties in developing the Company's platform technology. Consequently, forward-looking statements should be regarded solely as the Company's current plans, estimates and beliefs. Investors should not place undue reliance on forward-looking statements. The Company cannot guarantee future results, events, levels of activity, performance or achievements. The Company does not undertake and specifically declines any obligation to update, republish, or revise any forward-looking statements to reflect new information, future events or circumstances or to reflect the occurrences of unanticipated events, except as may be required by law.
Investor Contact
Jenene Thomas
Chief Executive Officer
JTC Team, LLC
T: 908.824.0775
[email protected]
Media Contact
Susan Roberts
T:202.779.0929
[email protected]
SOURCE: iTolerance, Inc.
View the original press release on ACCESS Newswire
A.Rodriguezv--AMWN