
-
 Gaza rescuers say Israeli forces kill 48 as ceasefire calls mount
Gaza rescuers say Israeli forces kill 48 as ceasefire calls mount
-
Sabalenka boosted by hitting with Djokovic and Sinner at Wimbledon

-
 Nigeria theme park offers escape from biting economy
Nigeria theme park offers escape from biting economy
-
Jury considers verdict in Sean Combs sex trafficking trial

-
 Wall Street stocks rally further on trade and tax deal optimism
Wall Street stocks rally further on trade and tax deal optimism
-
Sabalenka cruises on Wimbledon's hottest opening day as Alcaraz launches title bid

-
 Bosch breaks through as South Africa set Zimbabwe huge target
Bosch breaks through as South Africa set Zimbabwe huge target
-
S.Africa's ex-transport bosses charged over Zuma-era graft case

-
 'No panic' says Medvedev after shock Wimbledon exit
'No panic' says Medvedev after shock Wimbledon exit
-
Rescuers evacuate 50,000 as Turkey battles wildfires

-
 ADB acting on US concerns over China, bank chief tells AFP
ADB acting on US concerns over China, bank chief tells AFP
-
Archer misses out as England unchanged for second India Test

-
 US Senate begins nail-biting vote on Trump spending bill
US Senate begins nail-biting vote on Trump spending bill
-
Top seed Sabalenka cruises into Wimbledon second round

-
 Medvedev suffers shock early Wimbledon exit
Medvedev suffers shock early Wimbledon exit
-
Wall Street stocks rally further on trade deal optimism

-
 Britain's Tarvet says 'not here for the money' after landmark Wimbledon win
Britain's Tarvet says 'not here for the money' after landmark Wimbledon win
-
Tennis fans sizzle as heatwave hits Wimbledon

-
 Tearful Jabeur forced to retire from Wimbledon first-round clash
Tearful Jabeur forced to retire from Wimbledon first-round clash
-
No relief for Southern Europe as punishing heatwave persists

-
 PKK disarmament process to begin early July: report
PKK disarmament process to begin early July: report
-
Alcaraz, Sabalenka in action on day one at sizzling Wimbledon

-
 France court jails migrant smugglers over 2022 Channel deaths
France court jails migrant smugglers over 2022 Channel deaths
-
Stocks muted as investors eye US trade talks
-
 China says aircraft carriers conduct combat training in Pacific
China says aircraft carriers conduct combat training in Pacific
-
NGO loses bid to block UK export of military equipment to Israel

-
 Three talking points from Austrian Grand Prix
Three talking points from Austrian Grand Prix
-
Wimbledon 'ready' for soaring temperatures

-
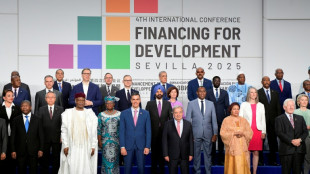 UN chief urges aid surge in world of 'climate chaos, raging conflicts'
UN chief urges aid surge in world of 'climate chaos, raging conflicts'
-
French injury worries mount ahead of first All Blacks Test

-
 India coach Gambhir faces growing pressure ahead of second England Test
India coach Gambhir faces growing pressure ahead of second England Test
-
Oasis ride Britpop revival as 90s make nostalgic comeback in UK

-
 'Embracing AI': TomTom cuts 300 jobs
'Embracing AI': TomTom cuts 300 jobs
-
'We have nothing': Afghans driven out of Iran return to uncertain future

-
 Bangladesh's biggest port resumes operations as strike ends
Bangladesh's biggest port resumes operations as strike ends
-
Havili, Frizell among All Blacks in Australia-NZ XV to face Lions

-
 Southern Europe roasts as temperatures soar
Southern Europe roasts as temperatures soar
-
Kenyan women jockey for place at DJ turntables

-
 Dalai Lama suggests institution to continue at 90th birthday launch
Dalai Lama suggests institution to continue at 90th birthday launch
-
Late fashion icon Lagerfeld's discreet villa near Paris goes under hammer

-
 Tougher Singapore crypto regulations kick in
Tougher Singapore crypto regulations kick in
-
Russia bets on patriotism to address demographic crisis

-
 Dalai Lama prays at landmark 90th birthday launch
Dalai Lama prays at landmark 90th birthday launch
-
India-Pakistan conflict hits shared love of film, music

-
 China's top diplomat visits Europe pitching closer ties in 'volatile' world
China's top diplomat visits Europe pitching closer ties in 'volatile' world
-
Kiss urges under-strength Reds to 'rip in' against Lions

-
 Canada rescinds tax on US tech firms in hopes of Trump trade deal
Canada rescinds tax on US tech firms in hopes of Trump trade deal
-
Most Asian stocks rise as investors eye US trade talks
-
 Jury retires to decide verdict in Australia's mushroom murder trial
Jury retires to decide verdict in Australia's mushroom murder trial
-
Farrell expects Reds to be 'big step up' for Lions


Moderna Announces Positive Phase 3 Results for Seasonal Influenza Vaccine
mRNA-1010 demonstrated superior relative vaccine efficacy that was 26.6% (95% CI; 16.7%, 35.4%) higher than a licensed standard-dose seasonal influenza vaccine in adults aged 50 years and older
CAMBRIDGE, MA / ACCESS Newswire / June 30, 2025 / Moderna, Inc. (NASDAQ:MRNA) today announced positive results from a Phase 3 efficacy study (P304) evaluating the relative vaccine efficacy (rVE) against influenza illness of mRNA-1010, the Company's seasonal influenza (flu) vaccine candidate, compared to a licensed standard-dose seasonal influenza vaccine in adults aged 50 years and older. mRNA-1010 achieved the most stringent superiority criterion prespecified in the protocol, with an rVE of 26.6% (95% CI; 16.7%, 35.4%) in the overall study population. Additionally, strong rVE was observed for each influenza strain contained in the vaccine, including A/H1N1 (rVE=29.6%), A/H3N2 (rVE=22.2%), and the B/Victoria lineages (rVE=29.1%). Subgroup analyses confirmed a consistently strong rVE point estimate across age groups, risk factors and previous influenza vaccination status. In participants aged 65 years and older, mRNA-1010 demonstrated an rVE of 27.4%.
"Today's strong Phase 3 efficacy results are a significant milestone in our effort to reduce the burden of influenza in older adults. The severity of this past flu season underscores the need for more effective vaccines," said Stéphane Bancel, Chief Executive Officer of Moderna. "An mRNA-based flu vaccine has the potential advantage to more precisely match circulating strains, support rapid response in a future influenza pandemic, and pave the way for COVID-19 combination vaccines."
In a previous Phase 3 study, mRNA-1010 had already demonstrated superior seroconversion rates and geometric mean titer ratios (GMR) against all strains included in the vaccine compared to both high-dose and standard-dose licensed seasonal influenza vaccine. [1]
According to the CDC, seasonal flu-related hospitalizations and outpatient visits reached a 15-year high during the 2024-2025 season. [2] More than 600,000 Americans were hospitalized due to flu-related illness last year, leading to substantial direct and indirect costs, as well as widespread disruption to daily life and work. [3]
P304 ( NCT06602024 ) is a Phase 3, randomized, observer-blind, active-controlled, case-driven, pivotal efficacy, immunogenicity and safety study. The trial enrolled 40,805 adults aged 50 years and older across 11 countries. Participants were randomly assigned to receive either a single dose of mRNA-1010 or a standard-dose licensed comparator, with a median follow-up of six months.
Safety and tolerability of mRNA-1010 were consistent with reported results from a previous Phase 3 study. [4] The majority of solicited adverse reactions (SARs) were mild. Injection site pain was the most common local SAR, and fatigue, headache and myalgia were the most common systemic SARs reported. There were no significant differences between the groups in the rates of unsolicited adverse events, serious adverse events, or adverse events of special interest.
Moderna plans to present these data at an upcoming medical conference and submit for peer-reviewed publication. The Company will engage with regulators on filing submissions for mRNA-1010.
About Moderna
Moderna is a leader in the creation of the field of mRNA medicine. Through the advancement of mRNA technology, Moderna is reimagining how medicines are made and transforming how we treat and prevent disease for everyone. By working at the intersection of science, technology and health for more than a decade, the company has developed medicines at unprecedented speed and efficiency, including one of the earliest and most effective COVID-19 vaccines.
Moderna's mRNA platform has enabled the development of therapeutics and vaccines for infectious diseases, immuno-oncology, rare diseases and autoimmune diseases. With a unique culture and a global team driven by the Moderna values and mindsets to responsibly change the future of human health, Moderna strives to deliver the greatest possible impact to people through mRNA medicines. For more information about Moderna, please visit modernatx.com and connect with us on X (formerly Twitter), Facebook, Instagram, YouTube and LinkedIn.
Forward-Looking Statements
This press release contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995, as amended, including statements regarding: Moderna's engagement with regulators on filing submissions for its standalone flu vaccine candidate; and the efficacy, safety and tolerability of mRNA-1010. The forward-looking statements in this press release are neither promises nor guarantees, and you should not place undue reliance on these forward-looking statements because they involve known and unknown risks, uncertainties, and other factors, many of which are beyond Moderna's control and which could cause actual results to differ materially from those expressed or implied by these forward-looking statements. These risks, uncertainties, and other factors include, among others, those risks and uncertainties described under the heading "Risk Factors" in Moderna's Annual Report on Form 10-K for the fiscal year ended December 31, 2024, and in subsequent filings made by Moderna with the U.S. Securities and Exchange Commission, which are available on the SEC's website at www.sec.gov . Except as required by law, Moderna disclaims any intention or responsibility for updating or revising any forward-looking statements contained in this press release in the event of new information, future developments or otherwise. These forward-looking statements are based on Moderna's current expectations and speak only as of the date of this press release.
Moderna Contacts
Media:
Chris Ridley
Head of Global Media Relations
+1 617-800-3651
[email protected]
Investors:
Lavina Talukdar
Senior Vice President & Head of Investor Relations
+1 617-209-5834
[email protected]
SOURCE: Moderna, Inc.
View the original press release on ACCESS Newswire
Y.Aukaiv--AMWN