
-
 Second seed Coco Gauff knocked out of Wimbledon
Second seed Coco Gauff knocked out of Wimbledon
-
Switzerland comes to the aid of Red Cross museum

-
 'That's life': No regrets for former champion Kvitova after Wimbledon farewell
'That's life': No regrets for former champion Kvitova after Wimbledon farewell
-
AI videos push Combs trial misinformation, researchers say

-
 UK govt guts key welfare reforms to win vote after internal rebellion
UK govt guts key welfare reforms to win vote after internal rebellion
-
Polish supreme court ratifies nationalist's presidential vote win

-
 Macron, Putin discuss Iran, Ukraine in first talks since 2022
Macron, Putin discuss Iran, Ukraine in first talks since 2022
-
French league launches own channel to broadcast Ligue 1

-
 Man City left to reflect on Club World Cup exit as tournament opens up
Man City left to reflect on Club World Cup exit as tournament opens up
-
Shock study: Mild electric stimulation boosts math ability

-
 Europe swelters as surprise early summer heatwave spreads
Europe swelters as surprise early summer heatwave spreads
-
Third seed Zverev stunned at Wimbledon

-
 Israel expands Gaza campaign ahead of Netanyahu's US visit
Israel expands Gaza campaign ahead of Netanyahu's US visit
-
Gaza mourns those killed in Israeli strike on seafront cafe

-
 Rubio hails end of USAID as Bush, Obama deplore cost in lives
Rubio hails end of USAID as Bush, Obama deplore cost in lives
-
Berlusconi family sell Monza football club to US investment fund

-
 UN aid meeting seeks end to Global South debt crisis
UN aid meeting seeks end to Global South debt crisis
-
Trump ramps up Musk feud with deportation threat

-
 French paparazzi boss handed 18-month suspended sentence for blackmail
French paparazzi boss handed 18-month suspended sentence for blackmail
-
Gilgeous-Alexander agrees record $285 mln extension: reports

-
 Tearful former champion Kvitova loses on Wimbledon farewell
Tearful former champion Kvitova loses on Wimbledon farewell
-
IMF urges Swiss to strengthen bank resilience

-
 Sri Lanka eye top-three spot in ODI rankings
Sri Lanka eye top-three spot in ODI rankings
-
Trump hails new 'Alligator Alcatraz' migrant detention center

-
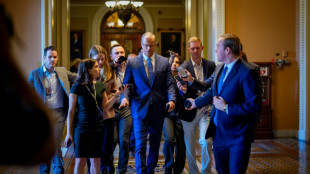 US Senate approves divisive Trump spending bill
US Senate approves divisive Trump spending bill
-
Krejcikova toughs it out in Wimbledon opener, Sinner cruises

-
 UK govt braces for crunch welfare reforms vote amid major rebellion
UK govt braces for crunch welfare reforms vote amid major rebellion
-
Shifting to Asia, Rubio meets Quad and talks minerals

-
 Stocks diverge while tracking US trade deal prospects
Stocks diverge while tracking US trade deal prospects
-
Bruce Lee Club closes archive doors citing operating costs

-
 Trump ramps up Musk feud with deportation, DOGE threats
Trump ramps up Musk feud with deportation, DOGE threats
-
BTS announces comeback for spring 2026

-
 Beating England without Bumrah 'not impossible' for India captain Gill
Beating England without Bumrah 'not impossible' for India captain Gill
-
Krejcikova battles back against rising star Eala to win Wimbledon opener

-
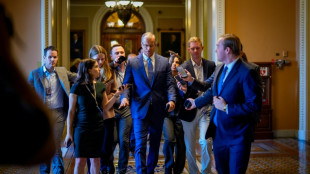 US Republicans close in on make-or-break Trump mega-bill vote
US Republicans close in on make-or-break Trump mega-bill vote
-
Arsenal sign goalkeeper Kepa from Chelsea

-
 Olympic champion Zheng knocked out of Wimbledon
Olympic champion Zheng knocked out of Wimbledon
-
Line judges missed at Wimbledon as AI takes their jobs

-
 Tshituka to make Test debut as Springboks change five
Tshituka to make Test debut as Springboks change five
-
'Remember Charlie Hebdo!' Protesters seethe at Istanbul magazine

-
 Top seed Sinner eases into Wimbledon second round
Top seed Sinner eases into Wimbledon second round
-
Stocks retreat as profit-taking follows Wall Street records

-
 Israel expands campaign in Gaza ahead of Netanyahu's US visit
Israel expands campaign in Gaza ahead of Netanyahu's US visit
-
Barcelona's Ansu Fati aims to kick-start career in Monaco

-
 Bordeaux-Begles drawn with Northampton in Champions Cup final repeat
Bordeaux-Begles drawn with Northampton in Champions Cup final repeat
-
Sean Combs trial: jurors seek verdict for a second day

-
 Trump says will 'take a look' at deporting Musk
Trump says will 'take a look' at deporting Musk
-
Greece starts charging tourist tax on cruises

-
 Trump heads for 'Alligator Alcatraz' migrant detention center
Trump heads for 'Alligator Alcatraz' migrant detention center
-
US Senate push to pass Trump's unpopular spending bill enters second day

ASTCT’s Leadership Contributes to FDA Decision to Eliminate REMS for BCMA- and CD19-Directed Autologous CAR T Cell Immunotherapies
CHICAGO, IL / ACCESS Newswire / July 1, 2025 / The U.S. Food and Drug Administration (FDA) has removed the Risk Evaluation and Mitigation Strategies (REMS) requirements for currently approved B-cell maturation antigen (BCMA)- and CD19-directed autologous chimeric antigen receptor (CAR) T cell therapies. The American Society for Transplantation and Cellular Therapy (ASTCT) contributed to this policy change, in part, by a 2023 multidisciplinary workshop hosted by ASTCT's 80/20 Subcommittee of the Committee on Cellular Therapy.
The workshop convened approximately 70 stakeholders-including clinicians from leading academic centers, regulators, accrediting organizations, professional societies, and immune effector cell (IEC) therapy manufacturers-to evaluate whether the field's existing safety and quality workflows could satisfy REMS requirements. The overwhelming consensus: current infrastructure and practices, supported by education and collaboration, provide a robust foundation for the continued safe use of CAR T therapies. Professional society guidelines, central accrediting, and auditing bodies (eg, FACT, NMDP BioTherapiesSM among others), and transparent data repositories (eg, CIBMTR®) can be leveraged to help ensure safety and quality practices in the absence of REMS requirements.
The workshop findings were communicated directly to the FDA during the Cell Therapy Liaison Meeting, presented at multiple professional society meetings and public regulatory forums and published in the Transplantation and Cellular Therapy journal.
The FDA has determined that REMS programs are no longer necessary to ensure the benefits of these CAR T cell immunotherapies outweigh their risks. This decision acknowledges the hematology/oncology community's extensive experience in managing risks such as cytokine release syndrome (CRS) and immune effector cell-associated neurotoxicity syndrome (ICANS).
The FDA concluded that the necessary safety information can be effectively conveyed through current product labeling-including boxed warnings-and Medication Guides, eliminating the need for separate REMS protocols that previously imposed operational burdens on treatment centers. In addition, time frames for monitoring following product administration were streamlined to make the clinical process more feasible for patients and families.
"We appreciate the FDA's decision recognizing the strength of our cell therapy community's clinical infrastructure. With elimination of the REMS requirements, we can reduce unnecessary administrative burden while maintaining the highest standards of patient safety. This will ultimately improve access to life-saving therapies" said Frederick L. Locke, MD, Chair of the Department of Blood and Marrow Transplant and Cellular Immunotherapy at Moffitt Cancer Center in Tampa FL, and Chair of the ASTCT Committee on Cellular Therapy."
"This announcement emphasizes the power of advocacy and collaboration between the FDA, accrediting bodies, professional societies and clinical sites in the cell therapy field." Sarah Nikiforow, MD, PhD, Cell Therapy Program at Dana-Farber Cancer Institute in Boston MA and cochair of the ASTCT 80/20 subcommittee.
###
About The American Society for Transplantation and Cellular Therapy® (ASTCT®):
The American Society for Transplantation and Cellular Therapy (ASTCT) is an international professional society dedicated to improving the lives of blood and marrow transplant and cellular therapy patients. ASTCT represents more than 3,900 physicians, investigators and other health care professionals from more than 45 countries. We are dedicated to improving the application and success of blood and marrow transplantation and related cellular therapies.
Contact Information
Jayne Kramer
Marketing Manager
[email protected]
202.367.2313
SOURCE: American Society for Transplantation and Cellular Therapy
View the original press release on ACCESS Newswire
Ch.Havering--AMWN
