
-
 Wimbledon line technology fails again as Fritz reaches semis
Wimbledon line technology fails again as Fritz reaches semis
-
Rubio imposter used AI to message high-level officials: report

-
 Kyiv, Moscow residents baffled by Trump's wavers on Ukraine aid
Kyiv, Moscow residents baffled by Trump's wavers on Ukraine aid
-
Archer can help England rattle impressive India, says Broad

-
 Iconic Bayeux Tapestry to be loaned to Britain: French president
Iconic Bayeux Tapestry to be loaned to Britain: French president
-
Lyles to make 200m return against Tebogo in Monaco

-
 UK post office scandal may have caused 13 suicides: inquiry
UK post office scandal may have caused 13 suicides: inquiry
-
Some Europeans still travel to Iran, ignoring dire warnings

-
 ICC seeks arrest of Taliban leaders over persecution of women
ICC seeks arrest of Taliban leaders over persecution of women
-
Stocks mark time as Trump postpones tariffs deadline

-
 India expect England's Archer to pose 'challenge'
India expect England's Archer to pose 'challenge'
-
Springboks make 11 changes for Italy Test

-
 Liverpool return to training in wake of Jota death
Liverpool return to training in wake of Jota death
-
France's Marseille airport says closing due to nearby wildfire

-
 France's Macron kicks off 'historic' UK state visit
France's Macron kicks off 'historic' UK state visit
-
Aussie prop Tupou hopes Racing move will bring smile back

-
 Speeding likely cause of Diogo Jota car crash: police
Speeding likely cause of Diogo Jota car crash: police
-
Bulgaria becomes 21st member to adopt euro after EU green light

-
 'Free culture': Slovak gunman defends Fico shooting as trial begins
'Free culture': Slovak gunman defends Fico shooting as trial begins
-
Rome to host Ukraine recovery conference as US support falters

-
 Qatar says 'we will need time' for Gaza ceasefire
Qatar says 'we will need time' for Gaza ceasefire
-
Alcaraz faces Norrie test at Wimbledon, Sabalenka eyes semi-finals

-
 Forest fire blazes in southern France
Forest fire blazes in southern France
-
Indian villagers beat five to death for 'witchcraft'

-
 Gaza ceasefire talks resume as Trump upbeat on deal
Gaza ceasefire talks resume as Trump upbeat on deal
-
Stocks rise as Trump delays tariffs deadline

-
 Acropolis shuts, outdoor work halted as heatwave scorches Greece
Acropolis shuts, outdoor work halted as heatwave scorches Greece
-
Newcastle agree £55m fee for Forest's Elanga - reports

-
 German exports to US tumble as Berlin urges quick trade deal
German exports to US tumble as Berlin urges quick trade deal
-
Tottenham sign Japan defender Takai

-
 Cambodian garment workers fret Trump's new tariff threat
Cambodian garment workers fret Trump's new tariff threat
-
Israel-Hamas ceasefire negotiations resume as Trump pushes for deal

-
 Trial of Slovak gunman who shot PM begins
Trial of Slovak gunman who shot PM begins
-
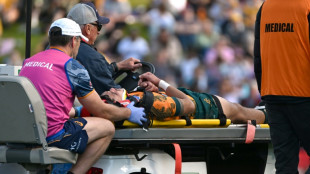
Wallabies' Lolesio faces long rehab after surgery
-
 Lions not invincible says former All Blacks coach Foster
Lions not invincible says former All Blacks coach Foster
-
Markets rise as Trump sends tariff letters, delays deadline

-
 Australia-born Lion Hansen faces 'pinch-me' moment against old team
Australia-born Lion Hansen faces 'pinch-me' moment against old team
-
Mitre by mitre: N. Macedonian nuns craft priceless holy headwear

-
 S.Leone islanders despair as rising ocean threatens survival
S.Leone islanders despair as rising ocean threatens survival
-
Bulgaria to get final green light to adopt euro in 2026

-
 Major garment producer Bangladesh seeks deal after 35% US tariff
Major garment producer Bangladesh seeks deal after 35% US tariff
-
France's Macron kicks off pomp-filled UK state visit

-
 Mbappe and PSG set for Club World Cup reunion as Real Madrid eye final
Mbappe and PSG set for Club World Cup reunion as Real Madrid eye final
-
US to send 'more weapons' to Ukraine: Trump

-
 Most markets rise as Trump sends tariff letters, delays deadline
Most markets rise as Trump sends tariff letters, delays deadline
-
Slovak gunman who shot PM to go on trial

-
 As heatwaves intensify, Morocco ups effort to warn residents
As heatwaves intensify, Morocco ups effort to warn residents
-
All Blacks captain Scott Barrett out for rest of France series

-
 AI video becomes more convincing, rattling creative industry
AI video becomes more convincing, rattling creative industry
-
Trump says new tariff deadline 'not 100 percent firm'


Dermata Receives Notice of Grant of Australian Patent for Topical Botulinum Toxin Treatment for Hyperhidrosis
- This is the Company's second granted patent for DMT410, using its Spongilla technology to topically deliver botulinum toxin for hyperhidrosis -
- The Company recently entered into a Clinical Trial Collaboration Agreement with Revance to study DMT410 for the treatment of axillary hyperhidrosis -
SAN DIEGO, CA / ACCESS Newswire / July 8, 2025 / Dermata Therapeutics, Inc. (NASDAQ:DRMA)(NASDAQ:DRMAW) ("Dermata" or the "Company"), a late-stage biotechnology company focusing on the treatment of medical skin diseases and aesthetic applications, today announced the Australian Patent Office has granted its patent application for its DMT410 program for the treatment of hyperhidrosis. The patent, entitled "Compositions for the treatment of skin conditions," (Australian Patent No. 2109284621) continues to strengthen Dermata's global intellectual property portfolio for DMT410 for the treatment of hyperhidrosis.
"We are thrilled to be granted this Australian patent, a testament to our team's relentless innovation and dedication to providing patients with unique and novel treatment options," said Gerry Proehl, Dermata's Chairman, President, and CEO. "We believe this patent reinforces our commitment to pushing boundaries for standard of care for hyperhidrosis patients and delivering groundbreaking solutions that we believe can transform the lives of patients. We are excited about the opportunities the DMT410 program can bring to patients, not only for the treatment of axillary hyperhidrosis, as we have seen in our proof-of-concept study, but potentially for palmer and plantar hyperhidrosis, which remain high unmet needs for patients," Mr. Proehl added.
About DMT410
DMT410 is the Company's combination treatment regimen that uses the unique mechanical features of the Company's XYNGARI™ product candidate to facilitate the intradermal delivery of botulinum toxin by topical application rather than through multiple injections with a needle. The treatment consists of an initial topical application of XYNGARI™ to the treatment area where the microscopic spicules penetrate the stratum corneum to create microchannels into the dermis allowing for the topical application and penetration of botulinum toxin. The Company has successfully completed proof-of-concept Phase 1 clinical trials using DMT410 in combination with BOTOX® for the treatment of primary axillary hyperhidrosis and for the treatment of multiple aesthetic skin conditions. Both studies showed promising efficacy results and appeared to be safe and well tolerated by patients. The Company is also planning to initiate a Phase 2a study of DMT410 (XYNGARI™ with DAXXIFY®) for the treatment of axillary hyperhidrosis.
About Dermata Therapeutics
Dermata Therapeutics is a late-stage biotechnology company focusing on the treatment of medical skin diseases and aesthetic applications. Dermata's lead product candidate, XYNGARI™ (formerly DMT310), is its first product candidate being developed from its Spongilla technology platform. XYNGARI™ is a once-weekly, topical product candidate derived from a naturally sourced freshwater sponge with multiple unique mechanisms of action that recently achieved positive data in a Phase 3 STAR-1 study for the treatment of moderate-to-severe acne. In addition to acne, XYNGARI™ has been studied for the treatment of psoriasis and rosacea. Dermata's second program, DMT410, uses XYNGARI™ as a new method for needle-free intradermal delivery of botulinum toxin for the treatment of multiple aesthetic and medical skin diseases and conditions. Dermata is headquartered in San Diego, California. For more information, please visit http://www.dermatarx.com/.
Forward-Looking Statements
Statements in this press release that are not strictly historical in nature are forward-looking statements. These statements are based on the Company's current beliefs and expectations and new risks may emerge from time to time. Forward-looking statements are subject to known and unknown risks, uncertainties, assumptions, and other factors including, but are not limited to, statements related to: expectations with regard to the timing of meetings and/or responses from submissions with regulatory bodies, including the FDA; the uncertainties inherent in clinical trials; expectations with regard to any potential partnership opportunities for any of the Company's product candidates; the Company's expectations with regard to current cash and cash equivalents and the amount of time it will fund operations; the success, cost, and timing of its product candidate DMT410 development activities and ongoing and planned clinical trials; whether the results of any planned clinical trials of DMT410 will lead to future product development; whether pending patent applications will proceed to allowance without interruption, if at all; and whether pending, accepted, or issued patents will provide adequate protection for the Company's product candidates, if approved. These statements are only predictions based on current information and expectations and involve a number of risks and uncertainties. Actual events or results may differ materially from those projected in any of such statements due to various factors, including the risks and uncertainties inherent in drug development, approval, and commercialization, and the fact that past results of clinical trials may not be indicative of future trial results. For a discussion of these and other factors, please refer to Dermata's filings with the Securities and Exchange Commission. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof. This caution is made under the safe harbor provisions of the Private Securities Litigation Reform Act of 1995. All forward-looking statements are qualified in their entirety by this cautionary statement and Dermata undertakes no obligation to revise or update this press release to reflect events or circumstances after the date hereof, except as required by law.
Investors:
Cliff Mastricola
Investor Relations
[email protected]
SOURCE: Dermata Therapeutics
View the original press release on ACCESS Newswire
H.E.Young--AMWN