
-
 Alleged Bondi shooters conducted 'tactical' training in countryside, Australian police say
Alleged Bondi shooters conducted 'tactical' training in countryside, Australian police say
-
Swiss court to hear landmark climate case against cement giant

-
 Knicks' Brunson scores 47, Bulls edge Hawks epic
Knicks' Brunson scores 47, Bulls edge Hawks epic
-
Global nuclear arms control under pressure in 2026

-
 Asian markets rally with Wall St as rate hopes rise, AI fears ease
Asian markets rally with Wall St as rate hopes rise, AI fears ease
-
Jailed Malaysian ex-PM Najib loses bid for house arrest

-
 Banned film exposes Hong Kong's censorship trend, director says
Banned film exposes Hong Kong's censorship trend, director says
-
Duffy, Patel force West Indies collapse as NZ close in on Test series win

-
 Australian state pushes tough gun laws, 'terror symbols' ban after shooting
Australian state pushes tough gun laws, 'terror symbols' ban after shooting
-
A night out on the town during Nigeria's 'Detty December'
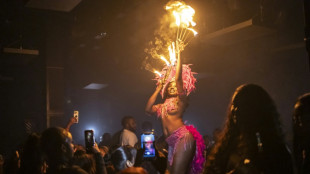
-
 US in 'pursuit' of third oil tanker in Caribbean: official
US in 'pursuit' of third oil tanker in Caribbean: official
-
CO2 soon to be buried under North Sea oil platform

-
 Steelers edge Lions as Bears, 49ers reach playoffs
Steelers edge Lions as Bears, 49ers reach playoffs
-
India's Bollywood counts costs as star fees squeeze profits

-
 McCullum admits errors in Ashes preparations as England look to salvage pride
McCullum admits errors in Ashes preparations as England look to salvage pride
-
Pets, pedis and peppermints: When the diva is a donkey

-
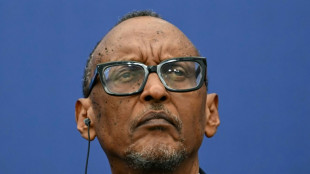 'A den of bandits': Rwanda closes thousands of evangelical churches
'A den of bandits': Rwanda closes thousands of evangelical churches
-
Southeast Asia bloc meets to press Thailand, Cambodia on truce

-
 As US battles China on AI, some companies choose Chinese
As US battles China on AI, some companies choose Chinese
-
AI resurrections of dead celebrities amuse and rankle
-
 Black Book Poll: "Governed AI" Emerges as the Deciding Factor in 2026 NHS Procurement
Black Book Poll: "Governed AI" Emerges as the Deciding Factor in 2026 NHS Procurement
-
Hemogenyx Pharmaceuticals PLC Announces Update on Admission of Shares

-
 Pantheon Resources PLC Announces Shareholder Letter and Corporate Update on Dubhe-1
Pantheon Resources PLC Announces Shareholder Letter and Corporate Update on Dubhe-1
-
Tocvan Begins Trenching Material for the Pilot Mine and Pushes Ahead With Infrastructure Development

-
 Steelers receiver Metcalf strikes Lions fan
Steelers receiver Metcalf strikes Lions fan
-
Morocco coach 'taking no risks' with Hakimi fitness

-
 Gang members given hundreds-years-long sentences in El Salvador
Gang members given hundreds-years-long sentences in El Salvador
-
Chargers, Bills edge closer to playoff berths

-
 Gang members given hundred-years-long sentences in El Salvador
Gang members given hundred-years-long sentences in El Salvador
-
Hosts Morocco off to winning start at Africa Cup of Nations

-
 No jacket required for Emery as Villa dream of title glory
No jacket required for Emery as Villa dream of title glory
-
Amorim fears United captain Fernandes will be out 'a while'

-
 Nigerian government frees 130 kidnapped Catholic schoolchildren
Nigerian government frees 130 kidnapped Catholic schoolchildren
-
Captain Kane helps undermanned Bayern go nine clear in Bundesliga

-
 Captain Kane helps undermanned Bayern go nine clear
Captain Kane helps undermanned Bayern go nine clear
-
Rogers stars as Villa beat Man Utd to boost title bid

-
 Barca strengthen Liga lead at Villarreal, Atletico go third
Barca strengthen Liga lead at Villarreal, Atletico go third
-
Third 'Avatar' film soars to top in N. American box office debut

-
 Third day of Ukraine settlement talks to begin in Miami
Third day of Ukraine settlement talks to begin in Miami
-
Barcelona's Raphinha, Yamal strike in Villarreal win

-
 Macron, on UAE visit, announces new French aircraft carrier
Macron, on UAE visit, announces new French aircraft carrier
-
Barca's Raphinha, Yamal strike in Villarreal win

-
 Gunmen kill 9, wound 10 in South Africa bar attack
Gunmen kill 9, wound 10 in South Africa bar attack
-
Allegations of new cover-up over Epstein files

-
 Atletico go third with comfortable win at Girona
Atletico go third with comfortable win at Girona
-
Schwarz breaks World Cup duck with Alta Badia giant slalom victory

-
 Salah unaffected by Liverpool turmoil ahead of AFCON opener - Egypt coach
Salah unaffected by Liverpool turmoil ahead of AFCON opener - Egypt coach
-
Goggia eases her pain with World Cup super-G win as Vonn takes third

-
 Goggia wins World Cup super-G as Vonn takes third
Goggia wins World Cup super-G as Vonn takes third
-
Cambodia says Thai border clashes displace over half a million


Moderna Receives Health Canada Approval for Updated COVID-19 Vaccine Targeting SARS-CoV-2 Variant LP.8.1
All 2025 pre-filled syringe doses to be made in Canada, marking a domestic production milestone
CAMBRIDGE, MA / ACCESS Newswire / August 22, 2025 / Moderna announced today that Health Canada has authorized its updated COVID-19 mRNA vaccine, Spikevax®, targeting the SARS-CoV-2 LP.8.1 variant, for individuals aged six months and older. Moderna is on track to deliver the updated vaccine in time for the 2025-2026 vaccination season.
All Spikevax pre-filled syringe (PFS) doses for the Canadian market will now be manufactured domestically, marking the first time Canada's entire PFS format is produced at home. The drug substance will be produced at Moderna's new facility in Laval, Quebec, with fill-finish operations completed by Novocol Pharma in Cambridge, Ontario. These Canadian-made doses are expected to be available for this fall.
"This approval is a regulatory milestone and a testament to Canada's growing leadership in biomanufacturing and public health resilience," said Stéphane Bancel, Chief Executive Officer of Moderna. "Thanks to Health Canada's timely and thorough review, we are proud to supply Spikevax doses to communities across the country, including, for the first time this year, doses produced in Canada."
Moderna's updated COVID-19 vaccine targeting LP.8.1 has already been granted approval by regulators in Europe, Japan, Switzerland and other countries. Additional regulatory applications are under review around the world in preparation for the coming season.
Access and Distribution
Eligibility for the public vaccination program is set by each province and territory. Those who meet provincial criteria will receive the vaccine free of charge. For individuals not covered under the public programs, efforts are ongoing with private insurers and payers to streamline access and reimbursement within the private sector.
Canadians are encouraged to consult their provincial or territorial health authorities for the latest information on eligibility and availability.
Regulatory Basis
Health Canada's authorization is based on a comprehensive body of evidence submitted by Moderna, including clinical, non-clinical, and real-world data supporting the vaccine's safety and efficacy.[1]
[1] Moderna Canada. SPIKEVAX® Product Monograph. August 21, 2025.
About Moderna
Moderna is a leader in the creation of the field of mRNA medicine. Through the advancement of mRNA technology, Moderna is reimagining how medicines are made and transforming how we treat and prevent disease for everyone. By working at the intersection of science, technology and health for more than a decade, the company has developed medicines at unprecedented speed and efficiency, including one of the earliest and most effective COVID-19 vaccines.
Moderna's mRNA platform has enabled the development of therapeutics and vaccines for infectious diseases, immuno-oncology, rare diseases and autoimmune diseases. With a unique culture and a global team driven by the Moderna values and mindsets to responsibly change the future of human health, Moderna strives to deliver the greatest possible impact to people through mRNA medicines. For more information about Moderna, please visit modernatx.com and connect with us on X (formerly Twitter), Facebook, Instagram, YouTube and LinkedIn.
Spikevax® is a registered trademark of Moderna.
Moderna Forward-Looking Statements
This press release contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995, as amended, including statements regarding: the availability of Moderna's updated COVID vaccine for the 2025-2026 vaccination season; Moderna's manufacturing; vaccine efficacy and safety; and Moderna's pending regulatory applications around the world. The forward-looking statements in this press release are neither promises nor guarantees, and you should not place undue reliance on these forward-looking statements because they involve known and unknown risks, uncertainties, and other factors, many of which are beyond Moderna's control and which could cause actual results to differ materially from those expressed or implied by these forward-looking statements. These risks, uncertainties, and other factors include, among others, those risks and uncertainties described under the heading "Risk Factors" in Moderna's Annual Report on Form 10-K for the fiscal year ended December 31, 2024, and in subsequent filings made by Moderna with the U.S. Securities and Exchange Commission, which are available on the SEC's website at www.sec.gov. Except as required by law, Moderna disclaims any intention or responsibility for updating or revising any forward-looking statements contained in this press release in the event of new information, future developments or otherwise. These forward-looking statements are based on Moderna's current expectations and speak only as of the date of this press release.
Moderna Contacts
Media:
Chris Ridley
Head of Global Media Relations
+1 617-800-3651
[email protected]
Investors:
Lavina Talukdar
Senior Vice President & Head of Investor Relations
+1 617-209-5834
[email protected]
SOURCE: Moderna, Inc.
View the original press release on ACCESS Newswire
L.Harper--AMWN