
-
 Alleged Bondi shooters conducted 'tactical' training in countryside, Australian police say
Alleged Bondi shooters conducted 'tactical' training in countryside, Australian police say
-
Swiss court to hear landmark climate case against cement giant

-
 Knicks' Brunson scores 47, Bulls edge Hawks epic
Knicks' Brunson scores 47, Bulls edge Hawks epic
-
Global nuclear arms control under pressure in 2026

-
 Asian markets rally with Wall St as rate hopes rise, AI fears ease
Asian markets rally with Wall St as rate hopes rise, AI fears ease
-
Jailed Malaysian ex-PM Najib loses bid for house arrest

-
 Banned film exposes Hong Kong's censorship trend, director says
Banned film exposes Hong Kong's censorship trend, director says
-
Duffy, Patel force West Indies collapse as NZ close in on Test series win

-
 Australian state pushes tough gun laws, 'terror symbols' ban after shooting
Australian state pushes tough gun laws, 'terror symbols' ban after shooting
-
A night out on the town during Nigeria's 'Detty December'
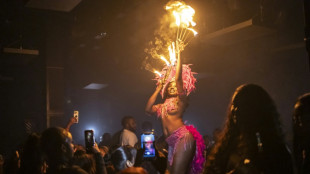
-
 US in 'pursuit' of third oil tanker in Caribbean: official
US in 'pursuit' of third oil tanker in Caribbean: official
-
CO2 soon to be buried under North Sea oil platform

-
 Steelers edge Lions as Bears, 49ers reach playoffs
Steelers edge Lions as Bears, 49ers reach playoffs
-
India's Bollywood counts costs as star fees squeeze profits

-
 McCullum admits errors in Ashes preparations as England look to salvage pride
McCullum admits errors in Ashes preparations as England look to salvage pride
-
Pets, pedis and peppermints: When the diva is a donkey

-
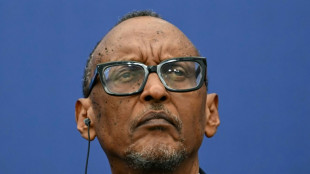 'A den of bandits': Rwanda closes thousands of evangelical churches
'A den of bandits': Rwanda closes thousands of evangelical churches
-
Southeast Asia bloc meets to press Thailand, Cambodia on truce

-
 As US battles China on AI, some companies choose Chinese
As US battles China on AI, some companies choose Chinese
-
AI resurrections of dead celebrities amuse and rankle
-
 Black Book Poll: "Governed AI" Emerges as the Deciding Factor in 2026 NHS Procurement
Black Book Poll: "Governed AI" Emerges as the Deciding Factor in 2026 NHS Procurement
-
Hemogenyx Pharmaceuticals PLC Announces Update on Admission of Shares

-
 Pantheon Resources PLC Announces Shareholder Letter and Corporate Update on Dubhe-1
Pantheon Resources PLC Announces Shareholder Letter and Corporate Update on Dubhe-1
-
Tocvan Begins Trenching Material for the Pilot Mine and Pushes Ahead With Infrastructure Development

-
 Steelers receiver Metcalf strikes Lions fan
Steelers receiver Metcalf strikes Lions fan
-
Morocco coach 'taking no risks' with Hakimi fitness

-
 Gang members given hundreds-years-long sentences in El Salvador
Gang members given hundreds-years-long sentences in El Salvador
-
Chargers, Bills edge closer to playoff berths

-
 Gang members given hundred-years-long sentences in El Salvador
Gang members given hundred-years-long sentences in El Salvador
-
Hosts Morocco off to winning start at Africa Cup of Nations

-
 No jacket required for Emery as Villa dream of title glory
No jacket required for Emery as Villa dream of title glory
-
Amorim fears United captain Fernandes will be out 'a while'

-
 Nigerian government frees 130 kidnapped Catholic schoolchildren
Nigerian government frees 130 kidnapped Catholic schoolchildren
-
Captain Kane helps undermanned Bayern go nine clear in Bundesliga

-
 Captain Kane helps undermanned Bayern go nine clear
Captain Kane helps undermanned Bayern go nine clear
-
Rogers stars as Villa beat Man Utd to boost title bid

-
 Barca strengthen Liga lead at Villarreal, Atletico go third
Barca strengthen Liga lead at Villarreal, Atletico go third
-
Third 'Avatar' film soars to top in N. American box office debut

-
 Third day of Ukraine settlement talks to begin in Miami
Third day of Ukraine settlement talks to begin in Miami
-
Barcelona's Raphinha, Yamal strike in Villarreal win

-
 Macron, on UAE visit, announces new French aircraft carrier
Macron, on UAE visit, announces new French aircraft carrier
-
Barca's Raphinha, Yamal strike in Villarreal win

-
 Gunmen kill 9, wound 10 in South Africa bar attack
Gunmen kill 9, wound 10 in South Africa bar attack
-
Allegations of new cover-up over Epstein files

-
 Atletico go third with comfortable win at Girona
Atletico go third with comfortable win at Girona
-
Schwarz breaks World Cup duck with Alta Badia giant slalom victory

-
 Salah unaffected by Liverpool turmoil ahead of AFCON opener - Egypt coach
Salah unaffected by Liverpool turmoil ahead of AFCON opener - Egypt coach
-
Goggia eases her pain with World Cup super-G win as Vonn takes third

-
 Goggia wins World Cup super-G as Vonn takes third
Goggia wins World Cup super-G as Vonn takes third
-
Cambodia says Thai border clashes displace over half a million


Trump Holds the Rescheduling Key: Will Marijuana Reform Follow the Patient's Right to Try Path?
From ‘Right to Try' to Cannabis: Trump's Next Breakthrough Could Unlock FDA Trials.
"President Trump's marijuana decision must go beyond politics-patients suffering from Huntington's disease and Multiple Sclerosis have waited nearly a decade for access to MMJ International Holdings' FDA-approved clinical trials. With capsules manufactured and science in place, all that's missing is the DEA stepping aside." stated Duane Boise, CEO MMJ International Holdings.
WASHINGTON, D.C. / ACCESS Newswire / August 24, 2025 / As national discourse intensifies around President Trump's forthcoming decision on marijuana rescheduling, MMJ International Holdings CEO Duane Boise urges the administration to finally align federal cannabis policy with science, patients, and the FDA drug approval process.

The conversation on drug reform is expanding beyond cannabis. Just this month, the Drug Enforcement Administration (DEA) requested a scientific review of psilocybin-a breakthrough for patients and advocates who have fought for decades to access novel therapies. With Health and Human Services Secretary Robert F. Kennedy Jr. signaling support for veterans' access to psychedelics, the political and scientific momentum for reform is undeniable.
For cannabis, however, progress has been stalled for years. President Biden ordered a review in 2022, HHS recommended Schedule III placement in 2023, yet the DEA has dragged its feet-continuing to block FDA approved clinical trials like those proposed by MMJ International Holdings for Huntington's disease and Multiple Sclerosis.
President Trump has the opportunity to change that. He already championed the Right to Try Act-granting terminally ill patients access to experimental treatments. The same principle should apply to cannabis science. If rescheduling moves forward, the nation must prioritize companies like MMJ International Holdings that have spent years building legitimate, FDA-compliant therapies.
"The President's decision can't just be about rescheduling-it must be about patients," said Duane Boise, President & CEO of MMJ International Holdings. "Just as the Right to Try law will break open access to innovative treatments, the marijuana fix should finally clear a path for FDA clinical trials that have been blocked for nearly a decade. MMJ is ready-we have the softgel capsules manufactured, the FDA orphan designations secured, and the science built. All we need is for the DEA to step aside and let the research happen."
Unlike state-legal operators chasing recreational markets, MMJ is pursuing the traditional pharmaceutical pathway. With two Investigational New Drug (IND) applications already filed, the company stands as the only U.S. entity prepared to conduct FDA-sanctioned clinical trials with cannabis-derived medicines.
As the nation awaits President Trump's announcement, MMJ International Holdings calls for immediate action:
Reschedule marijuana to Schedule III in line with HHS's recommendation.
Direct the DEA to stop obstructing federally compliant researchers like MMJ.
Prioritize FDA pathways over political gamesmanship to deliver real medicines to patients suffering from debilitating diseases.
About MMJ International Holdings
MMJ International Holdings, through its subsidiaries MMJ BioPharma Cultivation and MMJ BioPharma Labs, is a pioneering U.S.-based pharmaceutical company developing cannabis-derived therapies for Huntington's disease and Multiple Sclerosis. With FDA Orphan Drug Designations, manufactured softgel capsules, and INDs filed, MMJ is positioned to bring the first cannabis-based prescription medicines to market through the FDA approval process.
MMJ is represented by attorney Megan Sheehan.
CONTACT:
Madison Hisey
[email protected]
203-231-85832
SOURCE: MMJ International Holdings
View the original press release on ACCESS Newswire
A.Malone--AMWN