
-
 Alleged Bondi shooters conducted 'tactical' training in countryside, Australian police say
Alleged Bondi shooters conducted 'tactical' training in countryside, Australian police say
-
Swiss court to hear landmark climate case against cement giant

-
 Knicks' Brunson scores 47, Bulls edge Hawks epic
Knicks' Brunson scores 47, Bulls edge Hawks epic
-
Global nuclear arms control under pressure in 2026

-
 Asian markets rally with Wall St as rate hopes rise, AI fears ease
Asian markets rally with Wall St as rate hopes rise, AI fears ease
-
Jailed Malaysian ex-PM Najib loses bid for house arrest

-
 Banned film exposes Hong Kong's censorship trend, director says
Banned film exposes Hong Kong's censorship trend, director says
-
Duffy, Patel force West Indies collapse as NZ close in on Test series win

-
 Australian state pushes tough gun laws, 'terror symbols' ban after shooting
Australian state pushes tough gun laws, 'terror symbols' ban after shooting
-
A night out on the town during Nigeria's 'Detty December'
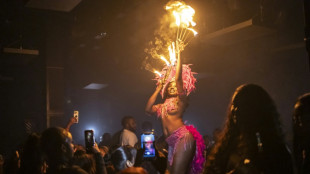
-
 US in 'pursuit' of third oil tanker in Caribbean: official
US in 'pursuit' of third oil tanker in Caribbean: official
-
CO2 soon to be buried under North Sea oil platform

-
 Steelers edge Lions as Bears, 49ers reach playoffs
Steelers edge Lions as Bears, 49ers reach playoffs
-
India's Bollywood counts costs as star fees squeeze profits

-
 McCullum admits errors in Ashes preparations as England look to salvage pride
McCullum admits errors in Ashes preparations as England look to salvage pride
-
Pets, pedis and peppermints: When the diva is a donkey

-
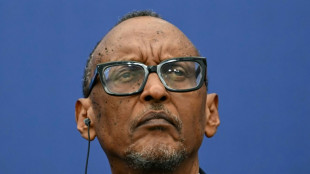 'A den of bandits': Rwanda closes thousands of evangelical churches
'A den of bandits': Rwanda closes thousands of evangelical churches
-
Southeast Asia bloc meets to press Thailand, Cambodia on truce

-
 As US battles China on AI, some companies choose Chinese
As US battles China on AI, some companies choose Chinese
-
AI resurrections of dead celebrities amuse and rankle
-
 Steelers receiver Metcalf strikes Lions fan
Steelers receiver Metcalf strikes Lions fan
-
Morocco coach 'taking no risks' with Hakimi fitness

-
 Gang members given hundreds-years-long sentences in El Salvador
Gang members given hundreds-years-long sentences in El Salvador
-
Chargers, Bills edge closer to playoff berths

-
 Gang members given hundred-years-long sentences in El Salvador
Gang members given hundred-years-long sentences in El Salvador
-
Hosts Morocco off to winning start at Africa Cup of Nations

-
 No jacket required for Emery as Villa dream of title glory
No jacket required for Emery as Villa dream of title glory
-
Amorim fears United captain Fernandes will be out 'a while'

-
 Nigerian government frees 130 kidnapped Catholic schoolchildren
Nigerian government frees 130 kidnapped Catholic schoolchildren
-
Captain Kane helps undermanned Bayern go nine clear in Bundesliga

-
 Captain Kane helps undermanned Bayern go nine clear
Captain Kane helps undermanned Bayern go nine clear
-
Rogers stars as Villa beat Man Utd to boost title bid

-
 Barca strengthen Liga lead at Villarreal, Atletico go third
Barca strengthen Liga lead at Villarreal, Atletico go third
-
Third 'Avatar' film soars to top in N. American box office debut

-
 Third day of Ukraine settlement talks to begin in Miami
Third day of Ukraine settlement talks to begin in Miami
-
Barcelona's Raphinha, Yamal strike in Villarreal win

-
 Macron, on UAE visit, announces new French aircraft carrier
Macron, on UAE visit, announces new French aircraft carrier
-
Barca's Raphinha, Yamal strike in Villarreal win

-
 Gunmen kill 9, wound 10 in South Africa bar attack
Gunmen kill 9, wound 10 in South Africa bar attack
-
Allegations of new cover-up over Epstein files

-
 Atletico go third with comfortable win at Girona
Atletico go third with comfortable win at Girona
-
Schwarz breaks World Cup duck with Alta Badia giant slalom victory

-
 Salah unaffected by Liverpool turmoil ahead of AFCON opener - Egypt coach
Salah unaffected by Liverpool turmoil ahead of AFCON opener - Egypt coach
-
Goggia eases her pain with World Cup super-G win as Vonn takes third

-
 Goggia wins World Cup super-G as Vonn takes third
Goggia wins World Cup super-G as Vonn takes third
-
Cambodia says Thai border clashes displace over half a million

-
 Kremlin denies three-way US-Ukraine-Russia talks in preparation
Kremlin denies three-way US-Ukraine-Russia talks in preparation
-
Williamson says 'series by series' call on New Zealand Test future

-
 Taiwan police rule out 'terrorism' in metro stabbing
Taiwan police rule out 'terrorism' in metro stabbing
-
Australia falls silent, lights candles for Bondi Beach shooting victims


RMTG Subsidiary Cellgenic to Host Soft Opening of Advanced Cell Therapy Manufacturing Lab in Cancun During September ISSCA Global Summit
RMTG Subsidiary Cellgenic to Host Soft Opening of Advanced Cell Therapy Manufacturing Lab in Cancun During September ISSCA Global Summit
COFEPRIS-Licensed Facility Positions Company for Full-Scale Operations by January 2026
LAS VEGAS, NV / ACCESS Newswire / August 25, 2025 / Regenerative Medical Technologies Group, Inc. (OTC:RMTG), a global leader in regenerative medicine solutions, today announced that its subsidiary Cellgenic will host the soft opening of its advanced cell therapy manufacturing facility in Cancun, Mexico during September 2025. The opening will coincide with the 2025 ISSCA Regenerative Medicine Global Summit, organized by the International Society for Stem Cell Application (ISSCA), RMTG's educational and scientific division through its Global Stem Cells Group subsidiary.
Strategic Manufacturing Infrastructure:
The soft opening represents a significant milestone in RMTG's international expansion strategy, with plans to establish specialized infrastructure designed for the controlled manufacture, preservation, and distribution of human cellular products. The strategically located Cancun facility will support production of investigational and regulated cell-based therapies, including mesenchymal stem cells (MSCs), exosomes, natural killer (NK) cells, and other biologics for orthopedics, neurology, pain management, aesthetic medicine, and longevity-focused therapies.
Regulatory Compliance and Market Positioning:
• COFEPRIS Authorization: Laboratory fully licensed by Mexico's federal health authority to operate as both a Stem Cell Collection Center and Stem Cell Bank
• International Standards: ISO-classified cleanrooms, cryopreservation systems, GMP-aligned workflows, and digital batch traceability systems
• Revenue Generation: Full-scale operations expected by January 2026, adding manufacturing revenue streams to RMTG's existing affiliate network spanning 30+ countries
• Training Integration: Designated areas for physician education programs through ISSCA platform, creating additional certification and training revenue opportunities
• Global Distribution: Facility positioned to serve physicians, clinics, and scientific partners both locally and internationally within regulatory framework
"This laboratory represents a pivotal step in our expansion strategy," said David Christensen, CEO of RMTG. "We believe it provides a foundation for scientific growth, and we are optimistic about its potential to support regulated, high-quality cell-based technologies for physicians and institutions worldwide."
Market Opportunity and Summit Integration:
The soft opening is expected to attract more than 200 physicians, scientists, and biotech leaders from over 30 countries attending the ISSCA Global Summit. The facility tour will provide attendees with an opportunity to observe manufacturing systems and quality safeguards, demonstrating RMTG's commitment to compliance and scientific rigor in the rapidly expanding regenerative medicine market.
"Our intention with this laboratory is to strengthen the infrastructure needed for responsible and scalable regenerative medicine," said Benito Novas, Founder of Global Stem Cells Group. "With the COFEPRIS authorizations in place, we believe this facility will serve as a strategic base to support the production and distribution of therapies that meet rigorous safety and quality expectations."
Operational Timeline and Revenue Impact:
Six months ago, we made the bold move to significantly expand our manufacturing operations. To do that we took the existing clinic and lab and separated the clinic to a new location, leaving that entire real estate footprint available for the necessary space required to increase our lab / manufacturing operation. That relocation of the clinic did cause a dip in procedures revenue, but we anticipate revenues to be strong again by the end of Q3.
The Cancun facility operates under Mexico's legal and biosafety framework for cellular product collection, processing, storage, and release. Full manufacturing operations beginning January 2026 will complement RMTG's existing revenue model, which generated Q1 2025 sales of $1.35M representing 67% growth with operational profits of $134,000. The facility supports RMTG's decentralized production strategy for faster, cost-effective delivery while helping partners navigate regulatory processes.
The laboratory will serve as a center for ongoing research, clinical collaboration, and physician education through ISSCA training programs. These programs support healthcare professionals in developing expertise with cell-based protocols while maintaining emphasis on ethics, compliance, and patient safety.
About RMTG:
RMTG operates through its Global Stem Cells Group subsidiary across more than 30 countries, distributing regenerative medicine solutions worldwide while specializing in physician education through its ISSCA platform. The Company's integrated approach combines clinical operations, product distribution, manufacturing capabilities, and medical education to capitalize on the rapidly expanding global regenerative medicine market. To learn more, visit www.stemcellsgroup.com
CAUTIONARY DISCLOSURE ABOUT FORWARD-LOOKING STATEMENTS
The information contained in this publication does not constitute an offer to sell or solicit an offer to buy securities of Regenerative Medical Technologies Group, Inc. (the "Company"). This publication contains forward-looking statements, which are not guarantees of future performance and may involve subjective judgment and analysis. As such, there are no assurances whatsoever that the Company will meet its expectations with respect to future revenues, manufacturing operations timeline, facility performance, or regulatory compliance. The information provided herein is believed to be accurate and reliable, however the Company makes no representations or warranties, expressed or implied, as to its accuracy or completeness. There is no guarantee that the Cellgenic facility will achieve full operational status by January 2026, that manufacturing operations will generate anticipated revenues, or that regulatory authorizations will remain in effect. The Company has no obligation to provide the recipient with additional updated information. No information in this publication should be interpreted as any indication whatsoever of the Company's future revenues, results of operations, or stock price.
Contact: David Christensen, CEO and President Regenerative Medical Technologies Group, Inc. [email protected] | (800) 956-3935
SOURCE: Regenerative Medical Technology Group
View the original press release on ACCESS Newswire
P.Mathewson--AMWN