
-
 Alleged Bondi shooters conducted 'tactical' training in countryside, Australian police say
Alleged Bondi shooters conducted 'tactical' training in countryside, Australian police say
-
Swiss court to hear landmark climate case against cement giant

-
 Knicks' Brunson scores 47, Bulls edge Hawks epic
Knicks' Brunson scores 47, Bulls edge Hawks epic
-
Global nuclear arms control under pressure in 2026

-
 Asian markets rally with Wall St as rate hopes rise, AI fears ease
Asian markets rally with Wall St as rate hopes rise, AI fears ease
-
Jailed Malaysian ex-PM Najib loses bid for house arrest

-
 Banned film exposes Hong Kong's censorship trend, director says
Banned film exposes Hong Kong's censorship trend, director says
-
Duffy, Patel force West Indies collapse as NZ close in on Test series win

-
 Australian state pushes tough gun laws, 'terror symbols' ban after shooting
Australian state pushes tough gun laws, 'terror symbols' ban after shooting
-
A night out on the town during Nigeria's 'Detty December'
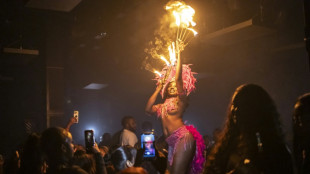
-
 US in 'pursuit' of third oil tanker in Caribbean: official
US in 'pursuit' of third oil tanker in Caribbean: official
-
CO2 soon to be buried under North Sea oil platform

-
 Steelers edge Lions as Bears, 49ers reach playoffs
Steelers edge Lions as Bears, 49ers reach playoffs
-
India's Bollywood counts costs as star fees squeeze profits

-
 McCullum admits errors in Ashes preparations as England look to salvage pride
McCullum admits errors in Ashes preparations as England look to salvage pride
-
Pets, pedis and peppermints: When the diva is a donkey

-
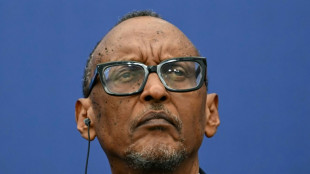 'A den of bandits': Rwanda closes thousands of evangelical churches
'A den of bandits': Rwanda closes thousands of evangelical churches
-
Southeast Asia bloc meets to press Thailand, Cambodia on truce

-
 As US battles China on AI, some companies choose Chinese
As US battles China on AI, some companies choose Chinese
-
AI resurrections of dead celebrities amuse and rankle
-
 Steelers receiver Metcalf strikes Lions fan
Steelers receiver Metcalf strikes Lions fan
-
Morocco coach 'taking no risks' with Hakimi fitness

-
 Gang members given hundreds-years-long sentences in El Salvador
Gang members given hundreds-years-long sentences in El Salvador
-
Chargers, Bills edge closer to playoff berths

-
 Gang members given hundred-years-long sentences in El Salvador
Gang members given hundred-years-long sentences in El Salvador
-
Hosts Morocco off to winning start at Africa Cup of Nations

-
 No jacket required for Emery as Villa dream of title glory
No jacket required for Emery as Villa dream of title glory
-
Amorim fears United captain Fernandes will be out 'a while'

-
 Nigerian government frees 130 kidnapped Catholic schoolchildren
Nigerian government frees 130 kidnapped Catholic schoolchildren
-
Captain Kane helps undermanned Bayern go nine clear in Bundesliga

-
 Captain Kane helps undermanned Bayern go nine clear
Captain Kane helps undermanned Bayern go nine clear
-
Rogers stars as Villa beat Man Utd to boost title bid

-
 Barca strengthen Liga lead at Villarreal, Atletico go third
Barca strengthen Liga lead at Villarreal, Atletico go third
-
Third 'Avatar' film soars to top in N. American box office debut

-
 Third day of Ukraine settlement talks to begin in Miami
Third day of Ukraine settlement talks to begin in Miami
-
Barcelona's Raphinha, Yamal strike in Villarreal win

-
 Macron, on UAE visit, announces new French aircraft carrier
Macron, on UAE visit, announces new French aircraft carrier
-
Barca's Raphinha, Yamal strike in Villarreal win

-
 Gunmen kill 9, wound 10 in South Africa bar attack
Gunmen kill 9, wound 10 in South Africa bar attack
-
Allegations of new cover-up over Epstein files

-
 Atletico go third with comfortable win at Girona
Atletico go third with comfortable win at Girona
-
Schwarz breaks World Cup duck with Alta Badia giant slalom victory

-
 Salah unaffected by Liverpool turmoil ahead of AFCON opener - Egypt coach
Salah unaffected by Liverpool turmoil ahead of AFCON opener - Egypt coach
-
Goggia eases her pain with World Cup super-G win as Vonn takes third

-
 Goggia wins World Cup super-G as Vonn takes third
Goggia wins World Cup super-G as Vonn takes third
-
Cambodia says Thai border clashes displace over half a million

-
 Kremlin denies three-way US-Ukraine-Russia talks in preparation
Kremlin denies three-way US-Ukraine-Russia talks in preparation
-
Williamson says 'series by series' call on New Zealand Test future

-
 Taiwan police rule out 'terrorism' in metro stabbing
Taiwan police rule out 'terrorism' in metro stabbing
-
Australia falls silent, lights candles for Bondi Beach shooting victims


Modular Medical Announces Completion of MODD1 Cartridge Production and Commencement of Pivot Conversion
-More than 6,000 MODD1 cartridges produced
- Conversion to Pivot-ready manufacturing in preparation for product launch in 1Q26
SAN DIEGO, CA / ACCESS Newswire / August 26, 2025 / Modular Medical, Inc. (Nasdaq:MODD) ("Modular Medical" or the "Company"), an insulin delivery technology company with the first FDA-cleared patch pump designed specifically to target all adult "almost-pumpers" with its user-friendly and affordable design, today announced that the MODD1 product cartridge production run has been completed and its manufacturing line is being converted to production for its Pivot product.
"I want to congratulate our operational team for the successful validation of our MODD1 manufacturing process and production of human use cartridges," said Jeb Besser, CEO of Modular Medical. We will now begin converting our cartridge line to Pivot production, and we expect it to be ready to produce cartridges for our Pivot product upon receipt of clearance from the U.S. Food and Drug Administration (the "FDA"). We expect to submit the Pivot product to the FDA for clearance in October 2025.
"The Pivot will be the first tubeless, removable 3 milliliter patch to be available to the consumer when it is cleared. Along with these features, the ability to scale production is a key differentiator in the pump space, especially given the much higher volumes required for a patch pump. Modular Medical's simple, low-cost platform was designed from the ground up for high volume manufacturing."
Forward-Looking Statements
This press release contains forward-looking statements that are made pursuant to the Safe Harbor provisions of the Private Securities Litigation Reform Act of 1995. Such forward-looking statements are subject to risks, trends, and uncertainties that could cause actual results to be materially different from the forward-looking statements contained in this press release, including, but not limited to, the timing of the Company's manufacturing line producing human-use cartridges for its Pivot product; the timing of the Company's submission of the Pivot product to the FDA; the Company's ability to convert patients to use its MODD1 pump; successful development of Modular Medical's proprietary technologies; whether the market will accept Modular Medical's products and services; anticipated consumer demand for the Company's products; whether Modular Medical can successfully manufacture its products at high volumes; the occurrence of future events or circumstances; general economic, and industry or political conditions in the United States or internationally; as well as other risk factors and business considerations described in Modular Medical's SEC filings, including its annual report on Form 10-K. Any forward-looking statements in this press release should be evaluated in light of these important risk factors. In addition, any forward-looking statements included in this press release represent Modular Medical's views only as of the date of its publication and should not be relied upon as representing its views as of any subsequent date. Modular Medical assumes no obligation to update these forward-looking statements, except as required by law.
About Modular Medical
Modular Medical, Inc. (Nasdaq:MODD) is a development-stage medical device company that intends to launch the next generation of insulin delivery technology. Using its patented technologies, the company seeks to eliminate the tradeoff between complexity and efficacy, thereby making top quality insulin delivery both affordable and simple to learn. Our mission is to improve access to the highest standard of glycemic control for people with diabetes taking it beyond "superusers" and providing "diabetes care for the rest of us."
Modular Medical was founded by Paul DiPerna, a seasoned medical device professional and microfluidics engineer. Prior to founding Modular Medical, Mr. DiPerna was the founder (in 2005) of Tandem Diabetes and invented and designed its t:slim insulin pump. More information is available at https://modular-medical.com.
All trademarks mentioned herein are the property of their respective owners.
CONTACT:
Jeb Besser
Chief Executive Officer
Modular Medical, Inc.
+1 (617) 399-1741
[email protected]
SOURCE: Modular Medical, Inc.
View the original press release on ACCESS Newswire
P.Stevenson--AMWN