
-
 Arsenal's Eze revels in 'special' hat-trick after destroying Spurs
Arsenal's Eze revels in 'special' hat-trick after destroying Spurs
-
Ouedraogo stunner sends Leipzig second in Bundesliga

-
 Ojomoh stars as England edge Argentina for 11th win in a row
Ojomoh stars as England edge Argentina for 11th win in a row
-
Eze treble fuels Arsenal's derby rout of Spurs

-
 'Wicked' sequel sees green in weekend-winning N. America debut
'Wicked' sequel sees green in weekend-winning N. America debut
-
Ojomoh stars as England edge Argentina in Twickenham thriller

-
 Israel says killed Hezbollah chief of staff in Beirut strike
Israel says killed Hezbollah chief of staff in Beirut strike
-
Roma take top spot in Serie A ahead of Milan derby

-
 Berrettini puts Italy on verge of third straight Davis Cup triumph
Berrettini puts Italy on verge of third straight Davis Cup triumph
-
Trump blasts Ukraine for 'zero gratitude' amid talks to halt war

-
 Ouedraogo stunner sends Leipzig second
Ouedraogo stunner sends Leipzig second
-
What does US 'terrorist' designation for Venezuela mean?

-
 Israel targets Hezbollah chief of staff in deadly Beirut strike
Israel targets Hezbollah chief of staff in deadly Beirut strike
-
Scotland thrash Tonga in Autumn Nations finale

-
 Three key Irish takeaways from Autumn Nations Series
Three key Irish takeaways from Autumn Nations Series
-
Imperious Shiffrin swoops to 103rd win at Gurgl

-
 Schmidt challenges Wallabies to 'roll up their sleeves' after gruesome year
Schmidt challenges Wallabies to 'roll up their sleeves' after gruesome year
-
Washington seeking to 'iron out' Trump proposal details with Ukrainians in Geneva

-
 South African centurion Muthusamy celebrates 'awesome' Test journey
South African centurion Muthusamy celebrates 'awesome' Test journey
-
Brazil 'very concerned' about US naval build-up near Venezuela

-
 Liverpool a 'mess' says Van Dijk
Liverpool a 'mess' says Van Dijk
-
First blind women's T20 cricket World Cup boosts sport

-
 France eye Dupont boost for Six Nations defence
France eye Dupont boost for Six Nations defence
-
McLaren boss apologises to Norris, Piastri for Vegas disqualification

-
 G20 grapples with splintering world order
G20 grapples with splintering world order
-
Verstappen wins big in Vegas with McLarens disqualified

-
 Muthusamy, Jansen put South Africa on top in second India Test
Muthusamy, Jansen put South Africa on top in second India Test
-
Rubio lands in Geneva for talks on Ukraine plan

-
 Norris and Piastri disqualified from Las Vegas GP
Norris and Piastri disqualified from Las Vegas GP
-
Slovenia holds crunch vote on contested assisted dying law

-
 Aonishiki beomes first Ukrainian to win sumo tournament
Aonishiki beomes first Ukrainian to win sumo tournament
-
Holders Australia drawn with New Zealand in Rugby League World Cup

-
 Vietnam flooding kills at least 90
Vietnam flooding kills at least 90
-
Muthusamy's maiden Test century powers South Africa to 428-7

-
 Myanmar junta says nearly 1,600 foreigners arrested in scam hub raids
Myanmar junta says nearly 1,600 foreigners arrested in scam hub raids
-
US signals room for negotiation on Ukraine plan ahead of talks

-
 Verstappen wins Las Vegas F1 Grand Prix, Norris edges closer to crown
Verstappen wins Las Vegas F1 Grand Prix, Norris edges closer to crown
-
Muthusamy anchors South Africa to 316-6 in second India Test

-
 Vietnam flood death toll rises to 90
Vietnam flood death toll rises to 90
-
US denies pushing Russian 'wish list' as Ukraine plan

-
 Harden's 55 leads Clippers win as Pistons streak hits 12
Harden's 55 leads Clippers win as Pistons streak hits 12
-
Kim's first top-10 in 14 years as Ballester wins maiden pro title

-
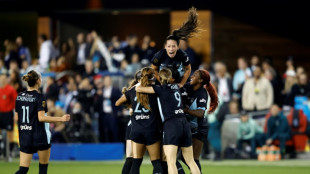 Gotham crowned NWSL champions after Lavelle breaks Spirit
Gotham crowned NWSL champions after Lavelle breaks Spirit
-
Trump signals room for negotiation on Ukraine plan ahead of talks

-
 Head shapes up as solution for Australia's opening woes
Head shapes up as solution for Australia's opening woes
-
Tomorrowland bets on Chinese dance music fans with first indoor event

-
 England slammed as 'brainless' after first Ashes Test capitulation
England slammed as 'brainless' after first Ashes Test capitulation
-
Slovenia to hold new vote on contested assisted dying law

-
 10 Benefits of Choosing Dental Implants After an Extraction
10 Benefits of Choosing Dental Implants After an Extraction
-
SKYLINE Announces Q3 2025 Financial Results


CASI Pharmaceuticals Receives and Appeals Delisting Determination from NASDAQ
SOUTH SAN FRANCISCO, CALIFORNIA / ACCESS Newswire / November 10, 2025 / CASI Pharmaceuticals, Inc. (NASDAQ:CASI), a clinical-stage biopharmaceutical company focused on developing CID-103, a potential best-in-class, anti-CD38 monoclonal antibody for patients with organ transplant rejection and autoimmune diseases, today announced that it has received a letter dated November 5, 2025, with a delisting determination from Nasdaq Stock Market LLC (the "Determination").
As previously reported, on May 5, 2025, Nasdaq notified the Company that its market value of listed securities (MVLS) had fallen below the minimum requirement of $35 million for 30 consecutive trading days, and as a result, did not comply with Listing Rule 5550(b)(2). The Company was provided 180 calendar days, or until November 3, 2025, to regain compliance with this rule. The Determination was made as the Company did not regain compliance for the extended compliance standard period set by Nasdaq during such 180-calendar-day grace period that ended on November 3, 2025.
CASI has already appealed this Determination and requested a hearing on the matter to present a detailed plan to Nasdaq to regain compliance, seeking a further extension of grace period to comply with the Nasdaq MVLS requirement. This appeal will stay the suspension of the Company's securities.
CASI remains committed to ensuring compliance and maintaining our Nasdaq listing. We are in constructive dialogue with Nasdaq regarding the process to regain compliance and will provide material updates to shareholders as they become available.
About CASI Pharmaceuticals
CASI Pharmaceuticals, Inc. is a public biopharmaceutical company developing CID-103, an anti-CD38 monoclonal antibody for organ transplant rejection and autoimmune diseases.
CID-103 is a fully human IgG1, potentially best-in-class, clinical stage, anti-CD38 monoclonal antibody which targets a unique epitope and has demonstrated an encouraging pre-clinical efficacy and clinical safety profile compared to other anti-CD38 monoclonal antibodies, and for which CASI owns exclusive global rights. CASI received FDA IND clearance to conduct a Phase 1 study in renal allograft antibody-mediated rejection (AMR) in the U.S. and plans for first patient in first quarter of 2026. In parallel, CASI is actively recruiting and dosing patients in an ongoing Phase 1 study in immune thrombocytopenia (ITP). In addition, CASI is assessing multiple technologies for development of a stable, high concentration protein solution for subcutaneous injection.
More information on CASI is available at www.casipharmaceuticals.com.
Forward Looking Statements
This announcement contains forward-looking statements. These statements are made under the "safe harbor" provisions of the U.S. Private Securities Litigation Reform Act of 1995. These forward-looking statements can be identified by terminology such as "will," "expects," "future," "intends," "plans," "believes," and similar statements. Among other things, the business outlook and quotations from management in this announcement, as well as the Company's strategic and operational plans, contain forward-looking statements. The Company may also make written or oral forward-looking statements in its periodic reports to the U.S. Securities and Exchange Commission (the "SEC"), in its annual report to shareholders, in press releases and other written materials and in oral statements made by its officers, directors or employees to third parties. Statements that are not historical facts, including statements about the Company's beliefs and expectations, are forward-looking statements. Forward-looking statements involve inherent risks and uncertainties. A number of factors could cause actual results to differ materially from those contained in any forward-looking statement, including but not limited to the following: the possibility that we may be delisted from trading on The Nasdaq Capital Market if we are not granted any extension of compliance period; uncertainties related to the possibility that the transaction for the divestiture of certain assets in China (the "Transaction") will not occur as planned if events arise that result in the termination of the Equity and Assets Transfer Agreement, or if one or more of the various closing conditions to the Transaction are not satisfied or waived; the possibility that our plan with respect to our business operations after the consummation of the Transaction can be implemented successfully; our recurring operating losses have raised substantial doubt regarding our ability to continue as a going concern; the volatility in the market price of our ordinary shares; the risk of substantial dilution of existing shareholders in future share issuances; the difficulty of executing our business strategy on a global basis including China; our inability to enter into strategic partnerships for the development, commercialization, manufacturing and distribution of our proposed product candidates or future candidates; legal or regulatory developments in China that adversely affect our ability to operate in China; our lack of experience in manufacturing products and uncertainty about our resources and capabilities to do so on a clinical or commercial scale; risks relating to the commercialization, if any, of our products and proposed products (such as marketing, safety, regulatory, patent, product liability, supply, competition and other risks); our inability to predict when or if our product candidates will be approved for marketing by the U.S. Food and Drug Administration, European Medicines Agency, PRC National Medical Products Administration, or other regulatory authorities; our inability to receive approval for renewal of license of our existing products; the risks relating to the need for additional capital and the uncertainty of securing additional funding on favorable terms; the risks associated with our product candidates, and the risks associated with our other early-stage products under development; the risk that result in preclinical and clinical models are not necessarily indicative of clinical results; uncertainties relating to preclinical and clinical trials, including delays to the commencement of such trials; our ability to protect our intellectual property rights; the lack of success in the clinical development of any of our products; and our dependence on third parties; the risks related to our dependence on Juventas to conduct the clinical development of CNCT19 and to partner with us to co-market CNCT19; risks related to our dependence on Juventas to ensure the patent protection and prosecution for CNCT19; the risk related to the Company's ongoing development of and regulatory application for CID-103 with respect to the treatment of antibody-mediated rejection for organ transplant and the license arrangements of CID-103; risks relating to interests of our largest shareholder and our Chairman that differ from our other shareholders; risks related to the development of a new manufacturing facility by CASI Pharmaceuticals (Wuxi) Co., Ltd.; and risks related to our disagreement with Acrotech with respect to the termination of agreements regarding EVOMELA®. Further information regarding these and other risks is included in the Company's filings with the SEC. All information provided herein is as of the date of this announcement, and the Company undertakes no obligation to update any forward-looking statement, except as required under applicable law. We caution readers not to place undue reliance on any forward-looking statements contained herein.
EVOMELA® is proprietary to Acrotech Biopharma Inc. and its affiliates. FOLOTYN®is proprietary to Acrotech Biopharma Inc and its affiliates. The Company is currently involved in disputes and legal proceedings related to certain pipeline products, including EVOMELA® and CNCT-19.Please refer to the Company's earlier SEC filing for further information.
INVESTOR CONTACT:
Ingrid Choong, PhD
650-619-6115
[email protected]
SOURCE: CASI Pharmaceuticals
View the original press release on ACCESS Newswire
P.Mathewson--AMWN

