
-
 Trump confirms call with Maduro, Caracas slams US maneuvers
Trump confirms call with Maduro, Caracas slams US maneuvers
-
Young dazzles as Panthers upset Rams, Bills down Steelers

-
 Arms makers see record revenues as tensions fuel demand: report
Arms makers see record revenues as tensions fuel demand: report
-
Trump optimistic after Ukraine talks as Rubio says 'more work' needed

-
 Real Madrid title hopes dented at Girona in third straight draw
Real Madrid title hopes dented at Girona in third straight draw
-
Pau beat La Rochelle as Hastoy sent off after 34 seconds

-
 Real Madrid drop points at Girona in third straight Liga draw
Real Madrid drop points at Girona in third straight Liga draw
-
Napoli beat rivals Roma to join Milan at Serie A summit

-
 Shiffrin bags 104th World Cup win with Copper Mountain slalom victory
Shiffrin bags 104th World Cup win with Copper Mountain slalom victory
-
Disney's 'Zootopia 2' rules Thanksgiving at N. American box office

-
 Arteta takes heart from Arsenal escape in Chelsea battle
Arteta takes heart from Arsenal escape in Chelsea battle
-
Duplantis and McLaughlin-Levrone crowned 'Athletes of the Year'

-
 Rubio says 'more work' required after US-Ukraine talks in Florida
Rubio says 'more work' required after US-Ukraine talks in Florida
-
McLaren boss admits team made strategy blunder

-
 West Ham's red-carded Paqueta slams FA for lack of support
West Ham's red-carded Paqueta slams FA for lack of support
-
Ramaphosa labels US attacks on S.Africa 'misinformation'

-
 Relaxed Verstappen set for another title showdown
Relaxed Verstappen set for another title showdown
-
Van Graan compares Bath match-winner Arundell to Springbok great Habana

-
 Arsenal held by 10-man Chelsea, Isak end drought to fire Liverpool
Arsenal held by 10-man Chelsea, Isak end drought to fire Liverpool
-
Slot hails 'important' Isak goal as Liverpool beat West Ham

-
 Merino strikes to give Arsenal bruising draw at 10-man Chelsea
Merino strikes to give Arsenal bruising draw at 10-man Chelsea
-
Thauvin double sends Lens top of Ligue 1 for 1st time in 21 years

-
 Pope urges Lebanese to embrace reconciliation, stay in crisis-hit country
Pope urges Lebanese to embrace reconciliation, stay in crisis-hit country
-
Arundell stars as Bath top Prem table with comeback win over Saracens

-
 Villarreal edge Real Sociedad, Betis win fiery derby
Villarreal edge Real Sociedad, Betis win fiery derby
-
Israel's Netanyahu seeks pardon in corruption cases

-
 Verstappen wins Qatar GP to set up final race title showdown
Verstappen wins Qatar GP to set up final race title showdown
-
Afghan suspect in Washington shooting likely radicalized in US: security official

-
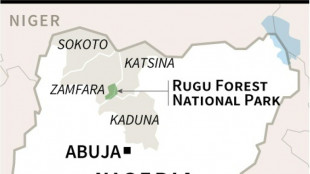 Pastor, bride among 26 kidnapped as Nigeria reels from raids
Pastor, bride among 26 kidnapped as Nigeria reels from raids
-
Trump officials host crucial Ukraine talks in Florida

-
 OPEC+ reaffirms planned pause on oil output hikes until March
OPEC+ reaffirms planned pause on oil output hikes until March
-
Kohli stars as India beat South Africa in first ODI

-
 Long-lost Rubens 'masterpiece' sells for almost 3 mn euros
Long-lost Rubens 'masterpiece' sells for almost 3 mn euros
-
Set-piece theft pays off for Man Utd: Amorim

-
 Isak scores first Premier League goal for Liverpool to sink West Ham
Isak scores first Premier League goal for Liverpool to sink West Ham
-
Death toll from Sri Lanka floods, landslides rises to 334: disaster agency

-
 Martinez double at Pisa keeps Inter on heels of Serie A leaders AC Milan
Martinez double at Pisa keeps Inter on heels of Serie A leaders AC Milan
-
Swiss reject compulsory civic duty, climate tax for super-rich

-
 Moleiro snatches Villarreal late winner at Real Sociedad
Moleiro snatches Villarreal late winner at Real Sociedad
-
Pope arrives in Lebanon with message of peace for crisis-hit country

-
 Celtic close on Scottish leaders Hearts after beating Hibs
Celtic close on Scottish leaders Hearts after beating Hibs
-
Swiss right-to-die group says founder dies by assisted suicide

-
 Zirkzee ends goal drought to inspire Man Utd victory at Palace
Zirkzee ends goal drought to inspire Man Utd victory at Palace
-
Trump threats dominate as Hondurans vote for president

-
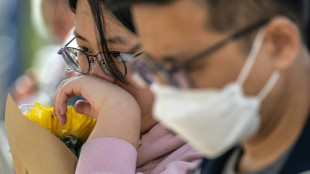 Hong Kong in mourning as fire death toll climbs to 146
Hong Kong in mourning as fire death toll climbs to 146
-
West Ham legend Bonds dies aged 79

-
 Swiss reject compulsory civic duty, climate tax for super-rich: projections
Swiss reject compulsory civic duty, climate tax for super-rich: projections
-
Kohli's 135 powers India to 349-8 in first South Africa ODI

-
 Indonesia, Thailand race to find missing as flooding toll tops 600
Indonesia, Thailand race to find missing as flooding toll tops 600
-
After call for Christian unity, pope leaves Turkey for Lebanon


MedMira Reports FY2025 Fourth Quarter and Year End Financial Results
HALIFAX, NOVA SCOTIA / ACCESS Newswire / November 28, 2025 / MedMira Inc. (MedMira) (TSXV:MIR), reported today on its financial results for the financial year ended July 31, 2025.
Corporate update
In FY2025, MedMira launched its recently Health Canada approved Multiplo® Rapid TP/HIV Test (Multiplo® TP/HIV) and its latest generation of its Reveal® Rapid HIV Test in Canada. Subsequent to FY2025, the Company received an additional approval for its Reveal® TP (Syphilis) rapid test by Health Canada. With this approval, the Company launched the fastest Syphilis antibody test available in Canada. With the support of MedMira's partners, provincial validation has been completed and the implementation of the Company's products the various provincial buying groups have commenced. This is a significant step for the Company to offer its products and allow health care professionals to gain access.
In the USA, the Company achieved VA and Federal listing that presents a major milestone to gain access to multi-year contracts and awards issued by these organizations. During FY2025, the Company further expanded its distribution network with specialized partners specifically aiming for VA, Federal and States contracts. These achievements enable MedMira to enhance its sales capabilities with established and preferred partners in the USA. Despite certain delays caused with the changing environment in the USA in the first part of the calendar year 2025, there has been a steady progress made in the second part of this year. In FY2025, the Company has been able to bid through its partners on these contracts with its current FDA approved Reveal® G4 HIV-1/2 rapid test and the awards and contract details will be made public when issued by the customers. It is the Company's anticipation with the future FDA CLIA waiver to gain further access to more contracts and further increase the overall sales opportunities.
In addition, the Company successfully completed the first phase of the clinical trials in Canada for its unique Multiplo® Complete Syphilis (TP/nTP) Antibody Test (Multiplo® TP/nTP). In early December 2025, an independent publication will be issued outlining the performance and positive impact of this product. Subsequent to FY2025, the Company started its second phase of the clinical trial with the anticipation to complete this in early Q1 2026. At the same time MedMira with its partners started the clinical trials for its Multiplo® TP/HIV rapid test for self-test use in Canada.
The Company has been working with its partners is on a structure to accelerate the commercial version of MedMira's unique MiROQ technology. MedMira's patented novel diagnostic system allows for accessible and efficient diagnostic tools for quantitative results in minutes. Especially in terms of cancer diagnostic this technology is able to make a significant impact on faster and more reliable diagnosis - when time is of essence.
At this stage, the Company is working on the commercial prototype and software development which will include AI as supporting element for future diagnostic. This strategic partnership will allow the Company to fast-track the development of this technology with the engagement of a strategic partner and with it allow an earlier market access. MiROQ enhances MedMira's RVF Technology and provides the opportunity to significantly expand its product offerings, market access and with it provide substantial value for the Company.
Profit and Loss Highlights
Revenue: The Company recorded sales and service revenues in FY2025 of $240,509 compared to $412,568 in FY2024.
Gross Profit: The Company recorded a gross profit in FY2025 of $172,851 compared to $226,489 for the same period last year. Gross profits were 72% in FY2025 compared to 55% in FY2024.
Operating expenses: In this financial year, the Company recorded operating expenses of $3,762,598 compared to $2,799,485 in FY2024. This increase is due to additional R&D projects and increased sales and marketing activities in preparation for new approvals.
Net loss: The Company recorded a net loss of $4,524,407 compared to $3,326,321 in FY2024.
Balance Sheet Highlights
Assets: The Company decreased its assets by $2,387,859 compared to last financial year which was mainly due to decreased cash on hand from funding its working capital requirements.
Liabilities: The Company's liabilities increased by $2,136,548 or 9% between FY2025 and FY2024. The increase was mainly due an increase in short term debt, trade payables and accrued liabilities.
Loans in default: the Company decreased its loans in default by $192,374 compared to the previous financial year. All other long and short terms debts are currently under negotiation to restructure terms and conditions of repayment.
Working Capital deficit: As a result of the increases above, the Company recorded a higher working capital deficit of $3,141,511 or 18% compared to last financial year.
The Company's financial statements and management's discussion and analysis are available on the Company's profile on SEDAR at www.sedar.com. For matters of going concern, reference is made to the Auditor's Emphasis of Matter statement in the fiscal year ended 2024 Auditors Report and note 2b in the audited financial statements which are also available on SEDAR.
About MedMira
MedMira is the developer and owner of Rapid Vertical Flow (RVF)® Technology. The Company's rapid test applications built on RVF Technology provide hospitals, labs, clinics and individuals with instant diagnosis for diseases such as HIV and hepatitis C in just three easy steps. The Company's tests are sold under the Reveal®, Multiplo® and Miriad® brands in global markets. MedMira's corporate offices and manufacturing facilities are located in Halifax, Nova Scotia, Canada and the Company has a sales and customer service office located in the United States. For more information visit medmira.com. Follow us on Twitter and LinkedIn.
This news release contains forward-looking statements, which involve risk and uncertainties and reflect the Company's current expectation regarding future events including statements regarding possible approval and launch of new products, future growth, and new business opportunities. Actual events could materially differ from those projected herein and depend on a number of factors including, but not limited to, changing market conditions, successful and timely completion of clinical studies, uncertainties related to the regulatory approval process, establishment of corporate alliances and other risks detailed from time to time in the company quarterly filings.
Neither TSX Venture Exchange nor its Regulation Services Provider (as that term is defined in the policies of the TSX Venture Exchange) accepts responsibility for the adequacy or accuracy of this release.
MedMira Contacts:
Markus Meile, CFO
Tel: 902-450-1588
Email: [email protected]
SOURCE: MedMira, Inc.
View the original press release on ACCESS Newswire
A.Mahlangu--AMWN