
-
 Starc becomes most prolific left-arm quick in Test history
Starc becomes most prolific left-arm quick in Test history
-
Keep energy infrastructure out of war, Turkey warns Moscow, Kyiv

-
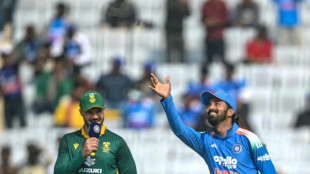 Coin toss curse puts India in a million-to-one heads or tailspin
Coin toss curse puts India in a million-to-one heads or tailspin
-
Asian markets mixed as traders struggle to hold Fed cut rally
-
 Crawley, Root guide England recovery after Starc's double strike
Crawley, Root guide England recovery after Starc's double strike
-
In Turkey, ancient carved faces shed new light on Neolithic society

-
 Eurovision members debate call to boycott Israel
Eurovision members debate call to boycott Israel
-
Ravindra, Latham tons put New Zealand in command of West Indies Test

-
 Seoul says six nationals held in North Korea, vows to help them
Seoul says six nationals held in North Korea, vows to help them
-
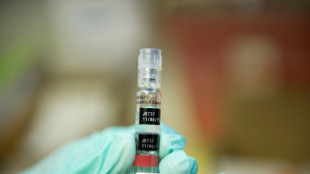
Hepatitis B vaccine for newborns faces scrutiny in US
-
 Most EU citizens see 'high risk' of war with Russia: poll
Most EU citizens see 'high risk' of war with Russia: poll
-
Ruthless Murray scores 52 to lead Nuggets, Bucks' Antetokounmpo injured

-
 Macron tells Xi China, France must overcome 'differences'
Macron tells Xi China, France must overcome 'differences'
-
Abu Dhabi showdown - how the F1 title can be won

-
 Daraya reborn: the rebels rebuilding Syria's deserted city
Daraya reborn: the rebels rebuilding Syria's deserted city
-
Rain forecasts raise fears in flood-hit Indonesia, Sri Lanka

-
 Tsunoda vows to return to F1 grid after 'tough' Red Bull axing
Tsunoda vows to return to F1 grid after 'tough' Red Bull axing
-
'No food': Indonesians scrounge for supplies after flood disaster

-
 Tree branches to fleece jackets: Chemicals plant in Germany bets on biomass
Tree branches to fleece jackets: Chemicals plant in Germany bets on biomass
-
Latham ton puts New Zealand firmly in charge of West Indies Test

-
 Asian markets stumble as traders struggle to hold Fed cut rally
Asian markets stumble as traders struggle to hold Fed cut rally
-
'Believe. Belong. Become': Brisbane 2032 Olympics unveils motto

-
 Florida's Venezuelans divided on US military buildup
Florida's Venezuelans divided on US military buildup
-
Norris faces nerve-shredding three-way scrap to claim maiden title

-
 Five of the best F1 last race title fights
Five of the best F1 last race title fights
-
Visa chaos and host city threats: how Trump disrupted World Cup plans

-
 France's Macron meets Xi for Ukraine, trade talks
France's Macron meets Xi for Ukraine, trade talks
-
Putin visits India for defence, trade talks

-
 Trump to sign Rwanda, DR Congo accord even as violence rages
Trump to sign Rwanda, DR Congo accord even as violence rages
-
Egypt's Sinai mountain megaproject threatens the people of St Catherine

-
 Nintendo launches long-awaited 'Metroid Prime 4' sci-fi blaster
Nintendo launches long-awaited 'Metroid Prime 4' sci-fi blaster
-
World Cup draw starts countdown to 2026 finals with Trump presiding

-
 All Blacks relishing prospect of South Africa clash at World Cup
All Blacks relishing prospect of South Africa clash at World Cup
-
France's Macron to meet Xi for Ukraine, trade talks

-
 Democrats release photos of Epstein's notorious private island
Democrats release photos of Epstein's notorious private island
-
Meta starts removing under-16s from social media in Australia

-
 New Zealand build 164-run lead but Windies claim Williamson
New Zealand build 164-run lead but Windies claim Williamson
-
Conor McGregor sexual assault lawsuit dropped

-
 Meta says starting to remove under-16s from social media in Australia
Meta says starting to remove under-16s from social media in Australia
-
Evotec-Partner Bayer Starts Phase 2 Study for Treatment of Patients with Alport Syndrome

-
 Grande Portage Resources Announces C$5Million Investment by Eric Sprott
Grande Portage Resources Announces C$5Million Investment by Eric Sprott
-
Tocvan Announces Positive Surface Results From North Block And Drill Program Update At Gran Pilar

-
 Liverpool fear factor gone, admits Slot
Liverpool fear factor gone, admits Slot
-
Maresca blasts 'very poor' Chelsea after damaging Leeds defeat

-
 Arteta fears injury woes will hamper Arsenal title charge
Arteta fears injury woes will hamper Arsenal title charge
-
Trump scraps Biden's fuel-economy standards, sparking climate outcry

-
 'Unbelievable' Merino strikes again to extend Arsenal's Premier League lead
'Unbelievable' Merino strikes again to extend Arsenal's Premier League lead
-
Doctor jailed for supplying ketamine to 'Friends' star Matthew Perry

-
 Arsenal extend Premier League lead, Chelsea beaten at Leeds
Arsenal extend Premier League lead, Chelsea beaten at Leeds
-
Chelsea's title challenge damaged by defeat at Leeds


Evotec-Partner Bayer Starts Phase 2 Study for Treatment of Patients with Alport Syndrome
Phase 2 clinical trial initiated to evaluate SEMA3A mAb as potential treatment for Alport syndrome
Milestone payment to Evotec expected upon first dosing of first study participant in early 2026
HAMBURG, DE / ACCESS Newswire / December 4, 2025 / Evotec SE (Frankfurt Stock Exchange:EVT, SDAX/TecDAX, Prime Standard, ISIN: DE0005664809, WKN 566480; NASDAQ:EVO) today announced that its partner Bayer AG has initiated a Phase 2 clinical study of a kidney disease program originating from the multi-target research collaboration between Evotec and Bayer in kidney diseases. Under the terms of the collaboration agreement, Evotec is eligible to receive a milestone payment upon first patient dosing, which is expected in early 2026. The study drug, BAY 3401016, a monoclonal antibody ("mAb") targeting the protein Semaphorin-3A ("Sema3A") is being developed as a potential treatment for Alport syndrome, a rare genetic kidney disease.
Bayer's ASSESS study is a randomized, double-blind, placebo-controlled, parallel group Phase 2a study with an extension phase to evaluate the efficacy and safety of BAY 3401016 in participants aged 18 to 45 with Alport syndrome. The program originates from a strategic collaboration, which Evotec and Bayer entered in August 2016. Under the terms of the agreement, Evotec is eligible to receive further development and sales milestones as well as tiered royalties of net sales contingent upon the future progress during clinical development and potential commercialization of a drug in the future.
Dr Cord Dohrmann, Chief Scientific Officer of Evotec, commented: "We are very pleased that our jointly developed antibody, BAY 3401016, for the treatment of Alport syndrome has advanced into Phase 2 of clinical development. Alport syndrome primarily damages the kidney, often starting at childhood and worsening through life. This debilitating disease significantly impacts patient's quality of life through both the symptoms and disease management, especially in later stages of kidney disease. New therapeutic options that enable better quality of life are urgently needed for individuals and families affected by this disease. The initiation of this study represents an important and hopeful step forward. We congratulate Bayer on the Phase 2 launch and are proud to support the advancement of this program."
About Semaphorin-3A
Semaphorin-3A ("Sema3A") is an extracellular guidance protein and a well-known regulator of the actin cytoskeleton. Alterations of the actin cytoskeleton, particularly of podocytes, are a key pathophysiological feature of Alport syndrome, a rare genetic kidney disease with progressive loss of filtration capacity, leading to end stage renal disease, progressive hearing loss and variable vision impairment. Sema3A is upregulated in injured human kidneys and implicated in the development and progression of acute and chronic kidney diseases. The monoclonal antibody ("mAb") developed by Bayer in partnership with Evotec blocks Sema3A activity and is currently investigated as a potential treatment of Alport syndrome, aiming to delay disease progression and onset of end-stage renal disease.
About Alport Syndrome
Alport syndrome is a genetic condition characterized by kidney disease, hearing loss, and eye abnormalities. Most affected individuals experience progressive loss of kidney function, which may lead to end-stage kidney disease. People with Alport syndrome also frequently develop sensorineural hearing loss in late childhood or early adolescence. The eye abnormalities characteristic of this condition seldom lead to vision loss. In 80% of cases, Alport syndrome is inherited in an X-linked manner and is caused by genetic changes in the COL4A5 gene. In the remaining cases, it may be inherited in either an autosomal recessive, or rarely in an autosomal dominant manner. In these cases, the condition is caused by genetic changes in the COL4A3 or COL4A4 genes. Diagnosis of the condition is based on family history of the condition, clinical signs, and specific testing such as a kidney biopsy. The diagnosis can be confirmed by genetic testing.
About Evotec SE
Evotec is a life science company that is pioneering the future of drug discovery and development. By integrating breakthrough science with AI-driven innovation and advanced technologies, we accelerate the journey from concept to cure - faster, smarter, and with greater precision.
Our expertise spans small molecules, biologics, cell therapies and associated modalities, supported by proprietary platforms such as Molecular Patient Databases, PanOmics and iPSC-based disease modeling.
With flexible partnering models tailored to our customers' needs, we work with all Top 20 Pharma companies, over 800 biotechs, academic institutions, and healthcare stakeholders. Our offerings range from standalone services to fully integrated R&D programs and long-term strategic partnerships, combining scientific excellence with operational agility.
Through Just - Evotec Biologics, we redefine biologics development and manufacturing to improve accessibility and affordability.
With a strong portfolio of over 100 proprietary R&D assets, most of them being co-owned, we focus on key therapeutic areas including oncology, cardiovascular and metabolic diseases, neurology, and immunology.
Evotec's global team of more than 4,800 experts operates from sites in Europe and the U.S., offering complementary technologies and services as synergistic centers of excellence. Learn more at www.evotec.com and follow us on LinkedIn and X/Twitter @Evotec
Forward-looking statements
This announcement contains forward-looking statements concerning future events, including the proposed offering and listing of Evotec's securities. Words such as "anticipate," "believe," "could," "estimate," "expect," "intend," "may," "might," "plan," "potential," "should," "target," "would" and variations of such words and similar expressions are intended to identify forward-looking statements. Such statements include comments regarding Evotec's expectations for revenues, Group EBITDA and unpartnered R&D expenses. These forward-looking statements are based on the information available to, and the expectations and assumptions deemed reasonable by Evotec at the time these statements were made. No assurance can be given that such expectations will prove to have been correct. These statements involve known and unknown risks and are based upon a number of assumptions and estimates, which are inherently subject to significant uncertainties and contingencies, many of which are beyond the control of Evotec. Evotec expressly disclaims any obligations or undertaking to release publicly any updates or revisions to any forward-looking statements contained herein to reflect any change in Evotec's expectations with respect thereto or any change in events, conditions or circumstances on which any statement is based.
For further information, please contact:
Media
Susanne Kreuter
VP Head of Strategic Marketing
[email protected]
Investor Relations
Volker Braun
EVP Head of Global Investor Relations & ESG
[email protected]
SOURCE: Evotec SE
View the original press release on ACCESS Newswire
G.Stevens--AMWN