
-
 Telefonica to shed around 5,500 jobs in Spain
Telefonica to shed around 5,500 jobs in Spain
-
McCullum wants to stay as England coach despite Ashes drubbing

-
 EU slams China dairy duties as 'unjustified'
EU slams China dairy duties as 'unjustified'
-
Italy fines Apple nearly 100 mn euros over app privacy feature

-
 America's Cup switches to two-year cycle
America's Cup switches to two-year cycle
-
Jesus could start for Arsenal in League Cup, says Arteta

-
 EU to probe Czech aid for two nuclear units
EU to probe Czech aid for two nuclear units
-
Strauss says sacking Stokes and McCullum will not solve England's Ashes woes

-
 Noel takes narrow lead after Alta Badia slalom first run
Noel takes narrow lead after Alta Badia slalom first run
-
Stocks diverge as rate hopes rise, AI fears ease

-
 Man City players face Christmas weigh-in as Guardiola issues 'fatty' warning
Man City players face Christmas weigh-in as Guardiola issues 'fatty' warning
-
German Christmas markets hit by flood of fake news

-
 Liverpool fear Isak has broken leg: reports
Liverpool fear Isak has broken leg: reports
-
West Indies captain says he 'let the team down' in New Zealand Tests

-
 Thailand says Cambodia agrees to border talks after ASEAN meet
Thailand says Cambodia agrees to border talks after ASEAN meet
-
Alleged Bondi shooters conducted 'tactical' training in countryside, Australian police say

-
 Swiss court to hear landmark climate case against cement giant
Swiss court to hear landmark climate case against cement giant
-
Knicks' Brunson scores 47, Bulls edge Hawks epic

-
 Global nuclear arms control under pressure in 2026
Global nuclear arms control under pressure in 2026
-
Asian markets rally with Wall St as rate hopes rise, AI fears ease

-
 Jailed Malaysian ex-PM Najib loses bid for house arrest
Jailed Malaysian ex-PM Najib loses bid for house arrest
-
Banned film exposes Hong Kong's censorship trend, director says

-
 Duffy, Patel force West Indies collapse as NZ close in on Test series win
Duffy, Patel force West Indies collapse as NZ close in on Test series win
-
Australian state pushes tough gun laws, 'terror symbols' ban after shooting

-
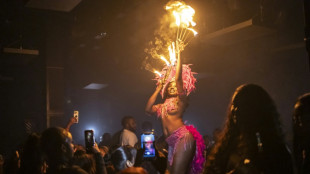 A night out on the town during Nigeria's 'Detty December'
A night out on the town during Nigeria's 'Detty December'
-
US in 'pursuit' of third oil tanker in Caribbean: official

-
 CO2 soon to be buried under North Sea oil platform
CO2 soon to be buried under North Sea oil platform
-
Steelers edge Lions as Bears, 49ers reach playoffs

-
 India's Bollywood counts costs as star fees squeeze profits
India's Bollywood counts costs as star fees squeeze profits
-
McCullum admits errors in Ashes preparations as England look to salvage pride

-
 Pets, pedis and peppermints: When the diva is a donkey
Pets, pedis and peppermints: When the diva is a donkey
-
'A den of bandits': Rwanda closes thousands of evangelical churches
-
 Southeast Asia bloc meets to press Thailand, Cambodia on truce
Southeast Asia bloc meets to press Thailand, Cambodia on truce
-
As US battles China on AI, some companies choose Chinese

-
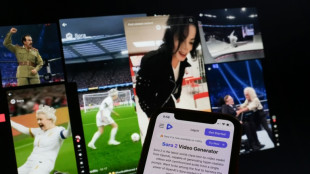 AI resurrections of dead celebrities amuse and rankle
AI resurrections of dead celebrities amuse and rankle
-
NESR Becomes First Oilfield Services Company to Commission Original Artwork Created from Recycled Produced Water
-
 SMX Strikes Joint Initiative with FinGo & Bougainville Refinery Ltd to Deliver Verifiable Identification for Trillion Dollar Gold Market
SMX Strikes Joint Initiative with FinGo & Bougainville Refinery Ltd to Deliver Verifiable Identification for Trillion Dollar Gold Market
-
Blue Gold and Trust Stamp Execute Strategic LOI to Develop Biometric, Passwordless Wallet Infrastructure for Gold-Backed Digital Assets

-
 SK tes Announces Grand Opening of New Shannon Facility, Marking a Milestone for Sustainable Technology in Ireland
SK tes Announces Grand Opening of New Shannon Facility, Marking a Milestone for Sustainable Technology in Ireland
-
FDA Officially Confirms Kava is a Food Under Federal Law

-
 Greenliant NVMe NANDrive(TM) SSDs Selected for Major Industrial, Aerospace and Mission Critical Programs
Greenliant NVMe NANDrive(TM) SSDs Selected for Major Industrial, Aerospace and Mission Critical Programs
-
World Renowned Law Firm Grant & Eisenhofer Files Class Action Lawsuit Against Canadian Banks CIBC and RBC Alleging Illegal Stock Market Manipulation of Quantum BioPharma Shares

-
 NextTrip Announces Pricing of Private Placement Financing of $3 Million
NextTrip Announces Pricing of Private Placement Financing of $3 Million
-
Namibia Critical Metals Inc. Receives Proceeds of $1,154,762 from Exercise of Warrants

-
 Shareholders Updates
Shareholders Updates
-
Applied Energetics Selected to Participate in Missile Defense Agency's Golden Dome (SHIELD) Multiple Award IDIQ Contract Vehicle

-
 Prospect Ridge Updates Diamond Drill Program at 100% Owned Camelot Copper-Gold Project in B.C.'S Cariboo Mining District
Prospect Ridge Updates Diamond Drill Program at 100% Owned Camelot Copper-Gold Project in B.C.'S Cariboo Mining District
-
The Alkaline Water Company Receives SEC Qualification of Tier 1 Regulation A Offering of Up to $10 Million

-
 Public Can Help Rid Oceans of Mines in New Freelancer Global Challenge
Public Can Help Rid Oceans of Mines in New Freelancer Global Challenge
-
Shareholders Update Report


Ovation Science Sees Expanded Opportunities for Its Topical Products Following U.S. Cannabis Rescheduling
VANCOUVER, BC AND LAS VEGAS, NV / ACCESS Newswire / December 22, 2025 / Ovation Science Inc. (CSE:OVAT)(OTC PINK:OVATF) ("Ovation" or the "Company") welcomes the recent U.S. rescheduling of cannabis and the potential introduction of a Medicare program that could allow patient access to much-needed, potentially life-changing CBD therapies, including topical formulations. Ovation's science-based topical / transdermal cannabis products are supported by clinical evidence and demonstrated commercial success through its licensee network.
This Executive Order to reclassify cannabis is expected to have a positive impact on Ovation by reducing regulatory barriers and increasing acceptance of cannabinoid-based products, particularly non-intoxicating topicals. Rescheduling supports broader research, clearer compliance pathways, and greater institutional and healthcare openness to CBD and other cannabinoid formulations, helping accelerate market adoption. For Ovation, this creates expanded opportunities for our science-backed topical products to reach patients and consumers seeking safe, effective, and innovative solutions, while strengthening partnerships, distribution, and long-term growth potential. Ovation is well positioned to benefit from this regulatory development, particularly its transdermal CBD topical products which are THC-free; meeting anticipated eligibility requirements for inclusion in a potential Medicare reimbursement program. Such a program could help seniors manage common health and wellness concerns and improve quality of life. Approximately 68 million Americans are enrolled in Medicare, with an estimated 35% experiencing inflammation-related conditions such as arthritis (Source: Centers for Medicare & Medicaid Services). While additional research is greatly needed, early studies suggest CBD may help reduce pain associated with inflammation, cancer and other conditions. Cannabis rescheduling may further enable expanded research, particularly in collaboration with government and academic institutions.
Ovation's topical formulations have demonstrated superior delivery of cannabinoids to and through the skin. In comparative laboratory testing, Ovation's topicals achieved CBD bioavailability of 82%, compared to just 2-3% for competing topical products. By contrast, oral CBD products, such as gummies, typically exhibit bioavailability of approximately 6%, as the compound must first pass through the liver ("first-pass metabolism"), where an estimated 70-75% of CBD is broken down before reaching systemic circulation. In addition to these challenges, CBD is known for poor water solubility and low stability. Ovation's proprietary Invisicare® drug delivery technology and formulations are designed to uniquely overcome these limitations.
"We are very encouraged by this transformative announcement from the White House and see it as a significant opportunity to license our topical products to companies seeking to expand into the U.S. market," said Terry Howlett, President of Ovation Science. "Our ongoing success with licensees in four states has already demonstrated strong demand for effective, science-based transdermal solutions. Licensees recognize the value of high-margin topical products that drive customer satisfaction and repeat purchasing. Greater regulatory flexibility around cannabis is expected to positively impact our financial results through potential Medicare reimbursement, expanded licensing opportunities, and increased research activity-creating long-term value for our shareholders and supporting continued investment in the cannabis industry."
Invisicare® Proven Technology Platform
Ovation's competitive advantage centers on its patented Invisicare® skin delivery system, with over two decades of pharmaceutical research. Laboratory testing demonstrated dramatically superior bioavailability providing substantially more CBD, THC and other cannabinoids effectively delivered to and through the skin using Ovation's Invisicare technology, ensuring consumers receive maximum therapeutic benefit.
Ovation continues to seek growth opportunities in both in the USA and internationally including Canada, Europe, and Australia.
Statements have not been evaluated by the Food and Drug Administration. These products are not intended to diagnose, treat, cure, or prevent any disease.
About Ovation Science Inc.
Ovation Science Inc. and its wholly-owned subsidiary; Ovation Science USA Inc., is a research and development company that develops topical and transdermal CBD/THC and other cannabinoid products which are out-licensed to licensed operators by state or country. Ovation also distributes topical CBD products under Ovation's own brands; ARLO CBD Beauty and InVibe® MD ("wellness" line); all powered by its patented Invisicare® skin delivery technology. Invisicare enhances the delivery of ingredients to and through the skin and is protected by patents and proprietary formulations which cannot be duplicated. With over 20 years of pharmaceutical drug delivery experience, Ovation's management and science team have created a unique pipeline of proprietary medical / wellness topical and transdermal products along with a line of anti-aging / beauty formulas. Ovation earns revenues from royalties on licensees' sales and the sale of Invisicare, along with revenue from its own product sales. Ovation has offices in Vancouver, Canada and Las Vegas, USA.
Stock symbols: CSE:OVAT OTC PINK:OVATF
Websites: CORPORATE: www.ovationscience.com
WELLNESS: www.invibemd.com BEAUTY: www.arlocbdbeauty.com
Forward-Looking Statements
Information set forth in this news release contains forward-looking statements that are based on assumptions as of the date of this news release. These statements reflect management's current estimates, beliefs, intentions and expectations and are subject to a number of risks and uncertainties that could cause actual results to differ materially from those described in the forward-looking statements. In particular there is no assurance of the impact on Ovation of the new legislation, or if there will be new research or licensing opportunities. Sales and acceptance of its products in any of the state or country cited, continued sales in dispensaries or in retail markets or expansion to other states or countries is not guaranteed. There are no guarantees of future performance or further changes to regulations. Ovation Science Inc. cautions that all forward-looking statements are inherently uncertain and that actual results may be affected by a number of material factors, many of which are beyond Ovation Science Inc.'s control. Accordingly, readers should not place undue reliance on the forward-looking information. Ovation disclaims any obligation to revise or update any such forward-looking information to reflect future results, events or circumstances, except as required by law.
Neither the Canadian Securities Exchange, OTC Markets nor its Regulation Services Provider accepts responsibility for the adequacy or accuracy of this release.
CORPORATE and LICENSING INQUIRIES:
Doreen McMorran: [email protected] PH: 604-982-5700
SOURCE: Ovation Science Inc.
View the original press release on ACCESS Newswire
A.Mahlangu--AMWN