
-
 'Girl with a Pearl Earring' to be shown in Japan, in rare trip abroad
'Girl with a Pearl Earring' to be shown in Japan, in rare trip abroad
-
Syria tells civilians to leave Aleppo's Kurdish areas

-
 'Sign of life': defence boom lifts German factory orders
'Sign of life': defence boom lifts German factory orders
-
Japan's Fast Retailing raises profit forecast after China growth

-
 Olympic champion Zheng out of Australian Open
Olympic champion Zheng out of Australian Open
-
England's Brook 'deeply sorry' for nightclub fracas

-
 New clashes in Iran as opposition urges more protests
New clashes in Iran as opposition urges more protests
-
Equity markets mostly down as traders eye US jobs data

-
 England cricket board launches immediate review into Ashes debacle
England cricket board launches immediate review into Ashes debacle
-
Dancing isn't enough: industry pushes for practical robots

-
 Asian markets mostly down as traders eye US jobs data
Asian markets mostly down as traders eye US jobs data
-
Australia to hold royal commission inquiry into Bondi Beach shooting

-
 Sabalenka accuses tour chiefs over 'insane' tennis schedule
Sabalenka accuses tour chiefs over 'insane' tennis schedule
-
Cambodia to liquidate bank founded by accused scam boss

-
 Farmers enter Paris on tractors in protest at trade deal
Farmers enter Paris on tractors in protest at trade deal
-
Viral 'Chinese Trump' wins laughs on both sides of Pacific

-
 Stokes vows to stay on but 'wrongs to put right' after crushing Ashes defeat
Stokes vows to stay on but 'wrongs to put right' after crushing Ashes defeat
-
Lidl to drop broadcast TV ads in France

-
 Stokes admits 'wrongs to put right' after crushing Ashes defeat
Stokes admits 'wrongs to put right' after crushing Ashes defeat
-
Sabalenka impresses again in Australian Open warm-up, vows more to come

-
 Gilgeous-Alexander to the rescue as Thunder sink Jazz in overtime
Gilgeous-Alexander to the rescue as Thunder sink Jazz in overtime
-
From Diaz to 'Mazadona' - five new faces starring at AFCON

-
 Startups go public in litmus test for Chinese AI
Startups go public in litmus test for Chinese AI
-
Death of Bazball: Five things we learned from Ashes series

-
 Australia's emotional Khawaja bows out for final time with Ashes win
Australia's emotional Khawaja bows out for final time with Ashes win
-
Asian markets mixed as traders eye US jobs data

-
 Australia win final Test to complete 4-1 Ashes triumph over England
Australia win final Test to complete 4-1 Ashes triumph over England
-
Trump withdraws US from key climate treaty, deepening global pullback

-
 Trump pulls US out of key climate treaty, deepening global pullback
Trump pulls US out of key climate treaty, deepening global pullback
-
Morocco under huge pressure as hosts face Cup of Nations heat

-
 Australia heatwave stokes risk of catastrophic bushfires
Australia heatwave stokes risk of catastrophic bushfires
-
Australia 71-2 at lunch, need 89 more to win final Ashes Test

-
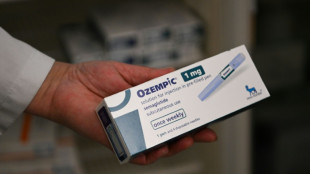 Study shows how fast kilos return after ending weight-loss drugs
Study shows how fast kilos return after ending weight-loss drugs
-
Trump pulls US out of key climate treaty, science body: White House

-
 England all out for 342, set Australia 160 to win final Ashes Test
England all out for 342, set Australia 160 to win final Ashes Test
-
FDA Clears Toetal Solution's Ziptoe Hammertoe System

-
 AbTherx and Red Queen Bio Partner on Therapeutic Antibody Discovery
AbTherx and Red Queen Bio Partner on Therapeutic Antibody Discovery
-
Banyan Gold Intersects Multiple High-Grade Mineralization Occurrences in Powerline and Airstrip Deposits, AurMac, Yukon, Canada

-
 Best Platform to Buy Crypto 2026 Announced
Best Platform to Buy Crypto 2026 Announced
-
New Digital Platform Fast Food Menu info Launches to Simplify Fast-Food Menu and Pricing Decisions

-
 Guam W2 Electronic Filing and EFW2 Generator for GuamTax.com W-2GU Wage Data Upload Announced by Real Business Solutions
Guam W2 Electronic Filing and EFW2 Generator for GuamTax.com W-2GU Wage Data Upload Announced by Real Business Solutions
-
Silver Range Provides an Operational Update

-
 Zenwork Joins the National Association of Computerized Tax Processors (NACTP)
Zenwork Joins the National Association of Computerized Tax Processors (NACTP)
-
Kee Ming Group Berhad Inks Underwriting Agreement With TA Securities Ahead Of ACE Market IPO

-
 Market Logic Announces Technology Partnership with Zappi to Accelerate Innovation with AI
Market Logic Announces Technology Partnership with Zappi to Accelerate Innovation with AI
-
Just - Evotec Biologics Receives Grant for AI-Driven Optimization of Monoclonal Antibody Developability for Affordable Access

-
 Lighthouse Names Georg Beyschlag Chief Financial Officer as Company Scales for Next Phase of Global Growth
Lighthouse Names Georg Beyschlag Chief Financial Officer as Company Scales for Next Phase of Global Growth
-
Storm in a tea cup for Frank as pressure mounts on Spurs boss

-
 Sesko spark masks Man Utd disappointment for Fletcher
Sesko spark masks Man Utd disappointment for Fletcher
-
Venezuelan opposition blindsided by Trump, waiting it out


Pentixapharm Receives FDA Feedback for Phase 3 Diagnostic Study in Hypertension
Formal written minutes from the U.S. FDA confirms no major concerns identified for the planned Phase 3 PANDA study of [⁶⁸Ga]Ga-PentixaFor in treatment-resistant hypertension and Primary Aldosteronism
Type B pre-IND meeting provided non-binding guidance on key statistical and methodological aspects, enabling refinement of the Phase 3 study design
Feedback clarifies evidence requirements relevant to a potential approval pathway, supporting the planned IND submission for the company's leading CXCR4-directed flagship program
BERLIN, DE / ACCESS Newswire / January 7, 2026 / Pentixapharm Holding AG (Frankfurt Prime Standard:PTP), an advanced clinical-stage biotech developing novel radiopharmaceuticals, today announced that it has received the formal written minutes from its recent scientific advice meeting with the U.S. Food and Drug Administration (FDA) for the planned Phase 3 PANDA study of [⁶⁸Ga]Ga-PentixaFor, the company's leading CXCR4-directed flagship program. The PANDA study will evaluate the radiodiagnostic as a potential new tool to improve the diagnostic pathway for patients with treatment-resistant hypertension and primary aldosteronism (PA). By enabling disease subtyping, PET/CT imaging with [⁶⁸Ga]Ga-PentixaFor has the potential to better guide the most appropriate therapy, supporting more precise and effective treatment decisions.
The FDA's minutes provide further clarification on several important statistical, methodological aspects, and criteria for assessing diagnostic performance of the planned Phase 3 study and expand on the preliminary feedback from the meeting, previously communicated on December 3, 2025 .
"The FDA's formal written feedback provides important clarity on key design and analysis elements of our planned Phase 3 PANDA study with [⁶⁸Ga]Ga-PentixaFor and confirms that no major concerns have been identified," said Dr. Dirk Pleimes, CEO and CMO of Pentixapharm. "Importantly, the feedback also provides guidance on the evidence requirements toward potential approval, including the role of confirmatory evidence in combination with this single Phase 3 study. This guidance strengthens our preparations for the planned IND submission and further supports the development of a potentially scalable, non-invasive diagnostic tool for patients with primary aldosteronism - the most common cause of secondary hypertension and a condition that remains significantly underdiagnosed."
About [ ⁶⁸Ga]Ga-PentixaForin treatment-resistant hypertension and primary aldosteronism
[⁶⁸Ga]Ga-PentixaFor is a novel, potentially first-in-class gallium-68-labeled radiodiagnostic designed to selectively target the chemokine receptor CXCR4 and to visualize its expression using high-resolution PET/CT imaging. Clinical experience with [⁶⁸Ga]Ga-PentixaFor PET/CT is documented in more than 100 scientific publications encompassing over 2,600 patients across multiple indications, including more than 1,600 patients with primary aldosteronism. In published and ongoing studies, the diagnostic has demonstrated reproducible in vivo imaging of CXCR4 expression with a favorable safety profile.
Recent research has shown strong CXCR4 overexpression in aldosterone-producing adrenal tumors, a hallmark of unilateral primary aldosteronism. Primary aldosteronism is a common but historically underdiagnosed cause of secondary hypertension, largely because reliably distinguishing unilateral from bilateral disease remains challenging with current diagnostic tools. Unilateral disease is typically treated by surgical removal of the affected adrenal gland whereas bilateral disease requires life-long medical therapy. By visualizing CXCR4 expression in aldosterone-producing tissue, [⁶⁸Ga]Ga-PentixaFor has the potential to support more reliable subtyping of primary aldosteronism and thereby better guide appropriate treatment decisions.
About Pentixapharm
Pentixapharm is an advanced clinical-stage biotech expanding the boundaries of radiopharmaceuticals. Headquartered in Berlin, Germany, the company develops precision diagnostics and therapeutics in oncology and cardiology to transform patient care. Its clinical pipeline is anchored by CXCR4-targeted PET-CT programs, including a Phase 3-ready candidate for the improved diagnosis of hypertensive patients with primary aldosteronism, which is intended to enable targeted treatment of the underlying causes of hypertension. CXCR4-based developments also include pioneering therapeutic programs in hematological cancers. Furthermore, Pentixapharm is advancing a next-generation antibody platform targeting CD24, an emerging immune-checkpoint marker over-expressed in multiple hard-to-treat cancers. Complemented by CXCR4 and CD24 intellectual property protection and a reliable isotope supply chain, Pentixapharm is poised to deliver meaningful patient benefit and sustainable growth in one of the fastest-growing areas of precision medicine.
Pentixapharm Investor and Media Contact
SOURCE: Pentixapharm Holding AG
View the original press release on ACCESS Newswire
M.A.Colin--AMWN
