-
 Former Montpellier coach Gasset dies at 72
Former Montpellier coach Gasset dies at 72
-
Trump's Christmas gospel: bombs, blessings and blame

-
 Russia lashes out at Zelensky ahead of new Trump meeting on Ukraine plan
Russia lashes out at Zelensky ahead of new Trump meeting on Ukraine plan
-
Salah helps Egypt beat South Africa and book last-16 place

-
 Australia's Ikitau facing lengthy lay-off after shoulder injury
Australia's Ikitau facing lengthy lay-off after shoulder injury
-
Another 1,100 refugees cross into Mauritania from Mali: UN

-
 Guardiola proud of Man City players' response to weighty issues
Guardiola proud of Man City players' response to weighty issues
-
Deadly blast hits mosque in Alawite area of Syria's Homs

-
 The Jukebox Man on song as Redknapp records 'dream' King George win
The Jukebox Man on song as Redknapp records 'dream' King George win
-
Liverpool boss Slot says Ekitike reaping rewards for greater physicality

-
 Judge jails ex-Malaysian PM Najib for 15 more years after new graft conviction
Judge jails ex-Malaysian PM Najib for 15 more years after new graft conviction
-
Musona rescues Zimbabwe in AFCON draw with Angola

-
 Zelensky to meet Trump in Florida on Sunday
Zelensky to meet Trump in Florida on Sunday
-
'Personality' the key for Celtic boss Nancy when it comes to new signings

-
 Arteta eager to avoid repeat of Rice red card against Brighton
Arteta eager to avoid repeat of Rice red card against Brighton
-
Nigeria signals more strikes likely in 'joint' US operations

-
 Malaysia's former PM Najib convicted in 1MDB graft trial
Malaysia's former PM Najib convicted in 1MDB graft trial
-
Elusive wild cat feared extinct rediscovered in Thailand

-
 Japan govt approves record budget, including for defence
Japan govt approves record budget, including for defence
-
Seoul to ease access to North Korean newspaper

-
 History-maker Tongue wants more of the same from England attack
History-maker Tongue wants more of the same from England attack
-
Australia lead England by 46 after 20 wickets fall on crazy day at MCG

-
 Asia markets edge up as precious metals surge
Asia markets edge up as precious metals surge
-
Twenty wickets fall on day one as Australia gain edge in 4th Ashes Test

-
 'No winner': Kosovo snap poll unlikely to end damaging deadlock
'No winner': Kosovo snap poll unlikely to end damaging deadlock
-
Culture being strangled by Kosovo's political crisis

-
 Main contenders in Kosovo's snap election
Main contenders in Kosovo's snap election
-
Australia all out for 152 as England take charge of 4th Ashes Test

-
 Boys recount 'torment' at hands of armed rebels in DR Congo
Boys recount 'torment' at hands of armed rebels in DR Congo
-
Inside Chernobyl, Ukraine scrambles to repair radiation shield

-
 Bondi victims honoured as Sydney-Hobart race sets sail
Bondi victims honoured as Sydney-Hobart race sets sail
-
North Korea's Kim orders factories to make more missiles in 2026

-
 Palladino's Atalanta on the up as Serie A leaders Inter visit
Palladino's Atalanta on the up as Serie A leaders Inter visit
-
Hooked on the claw: how crane games conquered Japan's arcades

-
 Shanghai's elderly waltz back to the past at lunchtime dance halls
Shanghai's elderly waltz back to the past at lunchtime dance halls
-
Japan govt approves record 122 trillion yen budget

-
 US launches Christmas Day strikes on IS targets in Nigeria
US launches Christmas Day strikes on IS targets in Nigeria
-
Australia reeling on 72-4 at lunch as England strike in 4th Ashes Test

-
 Too hot to handle? Searing heat looming over 2026 World Cup
Too hot to handle? Searing heat looming over 2026 World Cup
-
Packers clinch NFL playoff spot as Lions lose to Vikings

-
 Guinea's presidential candidates hold final rallies before Sunday's vote
Guinea's presidential candidates hold final rallies before Sunday's vote
-
President Trump's Executive Marijuana Action Exposes the Truth-How the DEA Delayed Medicine While Protecting Everything Else

-
 Calvin B. Taylor Bankshares, Inc. Reports Third Quarter Financial Results and Announces New Stock Repurchase Program
Calvin B. Taylor Bankshares, Inc. Reports Third Quarter Financial Results and Announces New Stock Repurchase Program
-
Processa Pharmaceuticals and 60 Degrees Pharmaceuticals Interviews to Air on the RedChip Small Stocks, Big Money(TM) Show on Bloomberg TV

-
 Aptevo Therapeutics Announces 1-for-18 Reverse Stock Split
Aptevo Therapeutics Announces 1-for-18 Reverse Stock Split
-
Loar Holdings Inc. Announced The Completion of its Acquisition of LMB Fans & Motors

-
 IRS Can Freeze Installment Agreements After Missed Filings - Clear Start Tax Explains Why Compliance Comes First
IRS Can Freeze Installment Agreements After Missed Filings - Clear Start Tax Explains Why Compliance Comes First
-
How the Terms of SMX's $111 Million Capital Facility Shape the Valuation Discussion

-
 A Christmas Message to the DEA's Diversion Anti Marijuana Cabal
A Christmas Message to the DEA's Diversion Anti Marijuana Cabal
-
QAT Community Sets QuantumTrade 5.0 for Public Beta Testing in March 2026


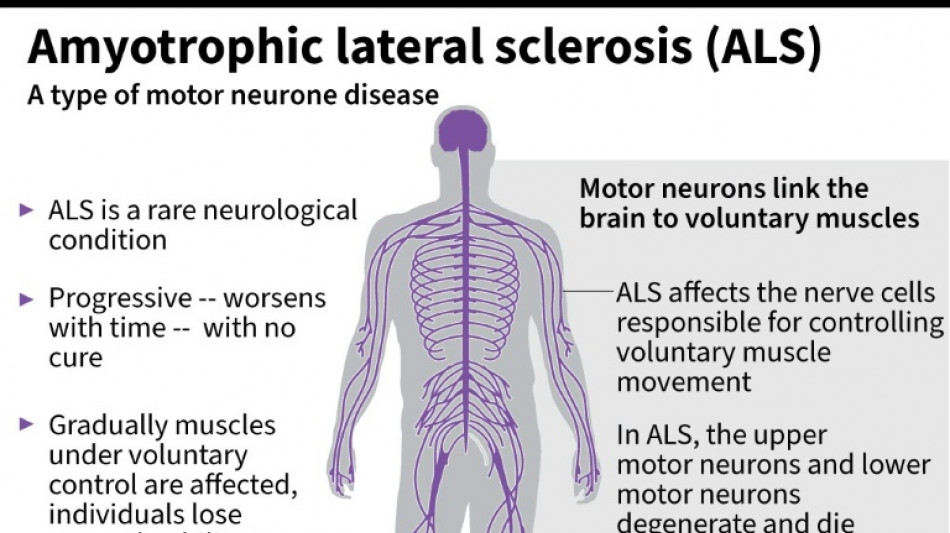
US company withdraws ALS drug after it fails in trial
Amylyx Pharmaceuticals announced Thursday it was withdrawing its approved treatment against the deadly neurodegenerative disease ALS after clinical data found no evidence the drug worked.
In a statement, the US company said it would discontinue its market authorizations for Relyvrio/Albrioza, using the brand names of the medicine in the US and Canadian markets.
"While this is a difficult moment for the ALS community, we reached this path forward in partnership with the stakeholders who will be impacted and in line with our steadfast commitment to people living with ALS and other neurodegenerative diseases," said the company's co-CEOs Joshua Cohen and Justin Klee in a statement.
The company also said it was reducing its workforce "by approximately 70 percent" as it focused on another experimental drug for use against ALS, and on repurposing Relyvrio for other conditions. It added it would continue to make Relyvrio available for patients who wish to keep using the treatment, through a "free drug program."
The news follows data from a clinical trial of 664 ALS patients announced in March, which found no significant differences in outcomes between those on the treatment group and those who received a placebo.
It was a big blow for patients with amyotrophic lateral sclerosis, sometimes called Lou Gehrig's disease after the famous baseball player, which devastates nerve cells in the brain and spinal cord.
ALS affects about two people per 100,000 every year, causing progressive loss of motor and cognitive function. Most patients die within five years of their diagnosis.
Relyvrio's approval by the US Food and Drug Administration in 2022 was controversial and based on the results of a single trial that involved just 137 participants.
The FDA itself noted there was "residual uncertainty about the evidence of effectiveness" -- but "given the serious and life-threatening nature of ALS and the substantial unmet need, this level of uncertainty is acceptable in this instance and consideration of these results in the context of regulatory flexibility is appropriate."
- Patient groups backed approval -
Advocacy groups also mounted a major campaign sending a petition to the FDA with tens of thousands of signatures urging approval. Once it became available, Amylyx reportedly announced an eye-watering list price of $158,000 per year in the US, drawing criticism.
Patient groups in Europe watched with desperation at the bureaucratic delays.
When the European Union drug watchdog later announced it was rejecting Relyvrio, the decision was slammed as "an affront" by angry French patients, who say they "don't have time to wait." France later relented, offering conditional approval in November.
"We commend Amylyx for pulling Relyvrio off the market, while still ensuring that people living with ALS can access the drug if they believe it is helping them," said the US-based ALS association, which had lobbied for the drug's approval and funded its research.
"Safe and potentially effective treatments can be made accessible rapidly until further research can confirm their efficacy," it added.
For now, there remain only a handful of treatments available.
Riluzole, FDA approved in 1995, prolongs life approximately three months. Edaravone, FDA approved in 2017, has been found to slow disease progression and improve survival.
And in 2023, the regulatory body approved tofersen, a gene therapy treatment that targets those ALS cases that are caused by mutations in the SOD1 gene.
Th.Berger--AMWN



