
-
 Sengun shines as Rockets rally to beat NBA champion Thunder
Sengun shines as Rockets rally to beat NBA champion Thunder
-
Matsuyama grabs PGA Phoenix Open lead with Hisatsune one back

-
 Washington Post CEO out after sweeping job cuts
Washington Post CEO out after sweeping job cuts
-
Haiti's transitional council hands power to PM

-
 N. Korea to hold party congress in February, first since 2021
N. Korea to hold party congress in February, first since 2021
-
Thailand votes after three leaders in two years

-
 Swiss joy as Von Allmen wins first gold of Winter Olympics
Swiss joy as Von Allmen wins first gold of Winter Olympics
-
George backs England to 'kick on' after Six Nations rout of Wales

-
 Malinin upstaged as Japan keep pressure on USA in skating team event
Malinin upstaged as Japan keep pressure on USA in skating team event
-
Vail's golden comets Vonn and Shiffrin inspire those who follow

-
 Veteran French politician loses culture post over Epstein links
Veteran French politician loses culture post over Epstein links
-
Japan's Kimura wins Olympic snowboard big air gold

-
 Arteta backs confident Gyokeres to hit 'highest level'
Arteta backs confident Gyokeres to hit 'highest level'
-
Hojlund the hero as Napoli snatch late win at Genoa

-
 England's Arundell 'frustrated' despite hat-trick in Wales romp
England's Arundell 'frustrated' despite hat-trick in Wales romp
-
Lollobrigida skates to first Italian gold of Winter Olympics on her birthday

-
 Arundell hat-trick inspires England thrashing of Wales in Six Nations opener
Arundell hat-trick inspires England thrashing of Wales in Six Nations opener
-
Chile's climate summit chief to lead plastic pollution treaty talks

-
 Rosenior hails 'unstoppable' Palmer after treble tames Wolves
Rosenior hails 'unstoppable' Palmer after treble tames Wolves
-
French ex-minister offers resignation from Paris cultural hub over Epstein links

-
 New NBA dunk contest champ assured and shooting stars return
New NBA dunk contest champ assured and shooting stars return
-
Shiffrin says will use lessons learnt from Beijing flop at 2026 Games

-
 Takaichi tipped for big win as Japan votes
Takaichi tipped for big win as Japan votes
-
Lens return top of Ligue 1 with win over Rennes

-
 Shiffrin learning from Beijing lessons ahead of Milan-Cortina bow
Shiffrin learning from Beijing lessons ahead of Milan-Cortina bow
-
Demonstrators in Berlin call for fall of Iran's Islamic republic

-
 'Free the mountains!": clashes at Milan protest over Winter Olympics
'Free the mountains!": clashes at Milan protest over Winter Olympics
-
Townsend accepts pressure will mount on him after Italy defeat

-
 BMW iX3 new style and design
BMW iX3 new style and design
-
Suryakumar's 84 leads India to opening win over USA in T20 World Cup

-
 Lollobrigida skates to first Italian gold of Milan-Cortina Games
Lollobrigida skates to first Italian gold of Milan-Cortina Games
-
Barca beat Mallorca to extend Liga lead

-
 Gyokeres lifts Arsenal nine clear as Man Utd pile pressure on Frank
Gyokeres lifts Arsenal nine clear as Man Utd pile pressure on Frank
-
Late Guirassy winner for Dortmund trims Bayern's lead atop Bundesliga

-
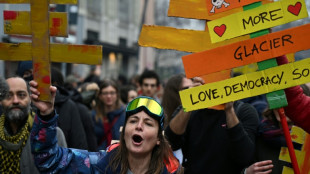 'Free the mountains!": protest in Milan over Winter Olympics
'Free the mountains!": protest in Milan over Winter Olympics
-
Gyokeres double helps Arsenal stretch Premier League lead

-
 New Skoda Epiq: modern with range
New Skoda Epiq: modern with range
-
Six Nations misery for Townsend as Italy beat sorry Scotland

-
 Spain, Portugal face fresh storms, torrential rain
Spain, Portugal face fresh storms, torrential rain
-
Opinions of Zuckerberg hang over social media addiction trial jury selection

-
 Over 2,200 IS detainees transferred to Iraq from Syria: Iraqi official
Over 2,200 IS detainees transferred to Iraq from Syria: Iraqi official
-
Norway's Ruud tops Olympic men's freeski slopestyle qualifying

-
 Czech qualifier Bejlek claims first title in Abu Dhabi
Czech qualifier Bejlek claims first title in Abu Dhabi
-
French duo reach Shanghai, completing year-and-a-half walk

-
 Australian snowboarder James eyes elusive Olympic gold
Australian snowboarder James eyes elusive Olympic gold
-
Sequins and snow: Eva Adamczykova makes Olympic return

-
 Vonn set for Olympic medal bid after successful downhill training
Vonn set for Olympic medal bid after successful downhill training
-
Shepherd takes hat-trick as West Indies beat Scotland in T20 World Cup

-
 Sausages will sell after thrill-seeker Von Allmen wins Olympic downhill
Sausages will sell after thrill-seeker Von Allmen wins Olympic downhill
-
Swiss racer Von Allmen wins first gold of Winter Olympics


Pfizer to seek US authorization for third Covid shot in children
Pfizer and BioNTech on Thursday announced positive results from a clinical trial on the safety and immune response of a third dose of their Covid vaccine in children aged five through 11, adding they would soon seek regulatory authorization.
Third doses of the vaccine are recommended for those aged 12 and up, and a fourth dose was recently recommended for people over 50.
Younger children -- except for those with immune compromising conditions -- have not been eligible for the third, making them more susceptible to infection from Omicron and its BA.2 subvariant.
BA.2 is now the globally dominant strain, and is behind a current spike in cases in the northeastern United States.
In the phase 2/3 trial, the companies analyzed data from 140 children aged five through 11, approximately six months after the second dose.
The dosage in this group is 10 micrograms, which was selected for safety reasons as children are more susceptible to side effects. The dose for those 12 and up is 30 micrograms.
Across the 140 children analyzed, the third dose was well tolerated, revealing no new safety concerns.
They also analyzed blood sera from a group of 30 individuals, finding that a third dose caused a 36-fold increase in levels of infection-blocking neutralizing antibodies against Omicron, compared to two doses.
Pfizer and BioNTech plan to soon submit the data to the US Food and Drug Administration (FDA), the European Medicines Agency and other regulatory agencies.
Most countries, including the United States, haven't yet authorized Covid vaccines for infants and very young children.
Last month, Moderna said it was pursuing approval for its vaccine in children aged six months through five years, using a two-dose regimen.
Pfizer's vaccine for this group was meant to be considered by the FDA in February but the agency postponed the meeting, because it wanted more data on how it would perform with three doses.
Ch.Havering--AMWN


