
-
 Pakistan's capital picks concrete over trees, angering residents
Pakistan's capital picks concrete over trees, angering residents
-
Berlin's crumbling 'Russian houses' trapped in bureaucratic limbo

-
 Neglected killer: kala-azar disease surges in Kenya
Neglected killer: kala-azar disease surges in Kenya
-
Super Bowl set for Patriots-Seahawks showdown as politics swirl

-
 Sengun shines as Rockets rally to beat NBA champion Thunder
Sengun shines as Rockets rally to beat NBA champion Thunder
-
Matsuyama grabs PGA Phoenix Open lead with Hisatsune one back

-
 Washington Post CEO out after sweeping job cuts
Washington Post CEO out after sweeping job cuts
-
Haiti's transitional council hands power to PM

-
 N. Korea to hold party congress in February, first since 2021
N. Korea to hold party congress in February, first since 2021
-
Thailand votes after three leaders in two years

-
 Swiss joy as Von Allmen wins first gold of Winter Olympics
Swiss joy as Von Allmen wins first gold of Winter Olympics
-
George backs England to 'kick on' after Six Nations rout of Wales

-
 Malinin upstaged as Japan keep pressure on USA in skating team event
Malinin upstaged as Japan keep pressure on USA in skating team event
-
Vail's golden comets Vonn and Shiffrin inspire those who follow

-
 Veteran French politician loses culture post over Epstein links
Veteran French politician loses culture post over Epstein links
-
Japan's Kimura wins Olympic snowboard big air gold

-
 Arteta backs confident Gyokeres to hit 'highest level'
Arteta backs confident Gyokeres to hit 'highest level'
-
Hojlund the hero as Napoli snatch late win at Genoa

-
 England's Arundell 'frustrated' despite hat-trick in Wales romp
England's Arundell 'frustrated' despite hat-trick in Wales romp
-
Lollobrigida skates to first Italian gold of Winter Olympics on her birthday

-
 Arundell hat-trick inspires England thrashing of Wales in Six Nations opener
Arundell hat-trick inspires England thrashing of Wales in Six Nations opener
-
Chile's climate summit chief to lead plastic pollution treaty talks

-
 Rosenior hails 'unstoppable' Palmer after treble tames Wolves
Rosenior hails 'unstoppable' Palmer after treble tames Wolves
-
French ex-minister offers resignation from Paris cultural hub over Epstein links

-
 New NBA dunk contest champ assured and shooting stars return
New NBA dunk contest champ assured and shooting stars return
-
Shiffrin says will use lessons learnt from Beijing flop at 2026 Games

-
 Takaichi tipped for big win as Japan votes
Takaichi tipped for big win as Japan votes
-
Lens return top of Ligue 1 with win over Rennes

-
 Shiffrin learning from Beijing lessons ahead of Milan-Cortina bow
Shiffrin learning from Beijing lessons ahead of Milan-Cortina bow
-
Demonstrators in Berlin call for fall of Iran's Islamic republic

-
 'Free the mountains!": clashes at Milan protest over Winter Olympics
'Free the mountains!": clashes at Milan protest over Winter Olympics
-
Townsend accepts pressure will mount on him after Italy defeat

-
 BMW iX3 new style and design
BMW iX3 new style and design
-
Suryakumar's 84 leads India to opening win over USA in T20 World Cup

-
 Lollobrigida skates to first Italian gold of Milan-Cortina Games
Lollobrigida skates to first Italian gold of Milan-Cortina Games
-
Barca beat Mallorca to extend Liga lead

-
 Gyokeres lifts Arsenal nine clear as Man Utd pile pressure on Frank
Gyokeres lifts Arsenal nine clear as Man Utd pile pressure on Frank
-
Late Guirassy winner for Dortmund trims Bayern's lead atop Bundesliga

-
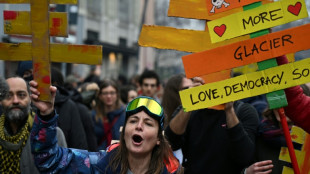 'Free the mountains!": protest in Milan over Winter Olympics
'Free the mountains!": protest in Milan over Winter Olympics
-
Gyokeres double helps Arsenal stretch Premier League lead

-
 New Skoda Epiq: modern with range
New Skoda Epiq: modern with range
-
Six Nations misery for Townsend as Italy beat sorry Scotland

-
 Spain, Portugal face fresh storms, torrential rain
Spain, Portugal face fresh storms, torrential rain
-
Opinions of Zuckerberg hang over social media addiction trial jury selection

-
 Over 2,200 IS detainees transferred to Iraq from Syria: Iraqi official
Over 2,200 IS detainees transferred to Iraq from Syria: Iraqi official
-
Norway's Ruud tops Olympic men's freeski slopestyle qualifying

-
 Czech qualifier Bejlek claims first title in Abu Dhabi
Czech qualifier Bejlek claims first title in Abu Dhabi
-
French duo reach Shanghai, completing year-and-a-half walk

-
 Australian snowboarder James eyes elusive Olympic gold
Australian snowboarder James eyes elusive Olympic gold
-
Sequins and snow: Eva Adamczykova makes Olympic return


US drug regulator schedules infant Covid vaccine meeting for June
The US Food and Drug Administration on Friday announced it would hold a set of meetings on Covid vaccines in June that would include deciding whether to authorize them for the youngest children.
Children five and under are the only group not yet eligible in the United States and most countries, a source of concern for many parents as infections are once more rising due to Omicron's subvariants.
The FDA -- considered the gold standard regulatory agency globally -- said in a statement it was calling panels of experts to discuss and likely vote on the Pfizer and Moderna vaccines on June 8, 21 and 22.
It was not clear on which dates the agency would consider Pfizer's application to authorize their vaccine in children six months through four years and Moderna's application to authorize their vaccine in children six months through five years.
Moderna is currently only approved for adults aged 18 and up and is also seeking authorization for ages six and up -- so one of the dates is reserved for this.
Of the two vaccines, Moderna appears slightly ahead, based on data announced so far.
Its two-dose regimen of 25 micrograms given to babies, toddlers and preschoolers generated similar levels of antibodies as two doses of 100 micrograms given to young people aged 18-25, indicating there would be similar levels of protection.
This was hailed as positive news by experts, who said it would help prevent severe disease, hospitalization, long-term consequences and death.
Pfizer's vaccine, dosed at three micrograms, did not meet its targets when given as two doses. The FDA subsequently asked for data on how it performed with three doses.
Even when they are unvaccinated, children under five are at very low risk for severe disease. There have been only 476 deaths in the United States this age group since the start of the pandemic, according to official data.
Among all US children, there have also been almost 8,000 cases of MIS-C, a post-viral inflammatory condition, that caused 66 deaths.
Separately, FDA panels will consider an application by Novavax for authorization in adults aged 18 and up for its protein subunit Covid vaccine.
On June 28, experts will consider whether the vaccines should be updated for new strains, and if so, which strains should be selected for this fall.
G.Stevens--AMWN


