-
 New Ypsilon and Ypsilon hf
New Ypsilon and Ypsilon hf
-
The Cupra Raval will be launched in 2026

-
 New id.Polo comes electric
New id.Polo comes electric
-
Iran defies US threats to insist on right to enrich uranium

-
 Seifert powers New Zealand to their record T20 World Cup chase
Seifert powers New Zealand to their record T20 World Cup chase
-
Naib's fifty lifts Afghanistan to 182-6 against New Zealand

-
 Paul Thomas Anderson wins top director prize for 'One Battle After Another'
Paul Thomas Anderson wins top director prize for 'One Battle After Another'
-
De Beers sale drags in diamond doldrums

-
 NFL embraces fashion as league seeks new audiences
NFL embraces fashion as league seeks new audiences
-
What's at stake for Indian agriculture in Trump's trade deal?

-
 Real Madrid can wait - Siraj's dream night after late T20 call-up
Real Madrid can wait - Siraj's dream night after late T20 call-up
-
Castle's monster night fuels Spurs, Rockets rally to beat Thunder
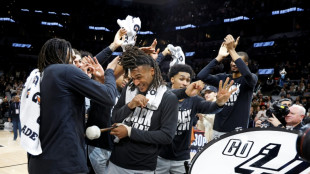
-
 Japan votes in snow-hit snap polls as Takaichi eyes strong mandate
Japan votes in snow-hit snap polls as Takaichi eyes strong mandate
-
Pakistan's capital picks concrete over trees, angering residents

-
 Berlin's crumbling 'Russian houses' trapped in bureaucratic limbo
Berlin's crumbling 'Russian houses' trapped in bureaucratic limbo
-
Neglected killer: kala-azar disease surges in Kenya

-
 Super Bowl set for Patriots-Seahawks showdown as politics swirl
Super Bowl set for Patriots-Seahawks showdown as politics swirl
-
Sengun shines as Rockets rally to beat NBA champion Thunder

-
 Matsuyama grabs PGA Phoenix Open lead with Hisatsune one back
Matsuyama grabs PGA Phoenix Open lead with Hisatsune one back
-
Washington Post CEO out after sweeping job cuts

-
 Haiti's transitional council hands power to PM
Haiti's transitional council hands power to PM
-
N. Korea to hold party congress in February, first since 2021

-
 Thailand votes after three leaders in two years
Thailand votes after three leaders in two years
-
Swiss joy as Von Allmen wins first gold of Winter Olympics

-
 George backs England to 'kick on' after Six Nations rout of Wales
George backs England to 'kick on' after Six Nations rout of Wales
-
Malinin upstaged as Japan keep pressure on USA in skating team event

-
 Vail's golden comets Vonn and Shiffrin inspire those who follow
Vail's golden comets Vonn and Shiffrin inspire those who follow
-
Veteran French politician loses culture post over Epstein links

-
 Japan's Kimura wins Olympic snowboard big air gold
Japan's Kimura wins Olympic snowboard big air gold
-
Arteta backs confident Gyokeres to hit 'highest level'

-
 Hojlund the hero as Napoli snatch late win at Genoa
Hojlund the hero as Napoli snatch late win at Genoa
-
England's Arundell 'frustrated' despite hat-trick in Wales romp

-
 Lollobrigida skates to first Italian gold of Winter Olympics on her birthday
Lollobrigida skates to first Italian gold of Winter Olympics on her birthday
-
Arundell hat-trick inspires England thrashing of Wales in Six Nations opener

-
 Chile's climate summit chief to lead plastic pollution treaty talks
Chile's climate summit chief to lead plastic pollution treaty talks
-
Rosenior hails 'unstoppable' Palmer after treble tames Wolves

-
 French ex-minister offers resignation from Paris cultural hub over Epstein links
French ex-minister offers resignation from Paris cultural hub over Epstein links
-
New NBA dunk contest champ assured and shooting stars return

-
 Shiffrin says will use lessons learnt from Beijing flop at 2026 Games
Shiffrin says will use lessons learnt from Beijing flop at 2026 Games
-
Takaichi tipped for big win as Japan votes

-
 Lens return top of Ligue 1 with win over Rennes
Lens return top of Ligue 1 with win over Rennes
-
Shiffrin learning from Beijing lessons ahead of Milan-Cortina bow

-
 Demonstrators in Berlin call for fall of Iran's Islamic republic
Demonstrators in Berlin call for fall of Iran's Islamic republic
-
'Free the mountains!": clashes at Milan protest over Winter Olympics

-
 Townsend accepts pressure will mount on him after Italy defeat
Townsend accepts pressure will mount on him after Italy defeat
-
BMW iX3 new style and design

-
 Suryakumar's 84 leads India to opening win over USA in T20 World Cup
Suryakumar's 84 leads India to opening win over USA in T20 World Cup
-
Lollobrigida skates to first Italian gold of Milan-Cortina Games

-
 Barca beat Mallorca to extend Liga lead
Barca beat Mallorca to extend Liga lead
-
Gyokeres lifts Arsenal nine clear as Man Utd pile pressure on Frank


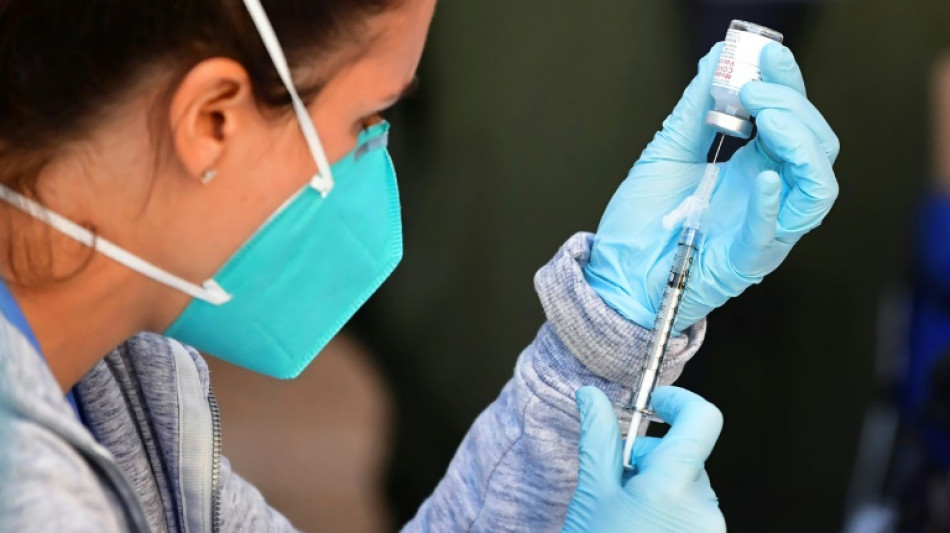
US approves Pfizer and Moderna vaccines for youngest children
The US Food and Drug Administration granted emergency authorization Friday for the use of Pfizer and Moderna Covid-19 vaccines in the youngest children, the final age group awaiting immunization in most countries.
The agency authorized Moderna's two-dose vaccine for children aged six months to five years, and three doses of Pfizer's shots for those between six months and four years old.
"Many parents, caregivers and clinicians have been waiting for a vaccine for younger children and this action will help protect those down to six months of age," Food and Drug Administration chief Robert Califf said in a statement.
"We expect that the vaccines for younger children will provide protection from the most severe outcomes of Covid-19, such as hospitalization and death."
The Centers for Disease Control and Prevention (CDC) must now also recommend the vaccines before they are put into use -- a final green light that will be given after a meeting of an advisory committee of experts that is expected to be held shortly.
But the US government has said that as soon as the FDA decision is made, 10 million doses could immediately be sent around the country, followed by millions more in subsequent weeks.
Both vaccines are based on messenger RNA, which delivers genetic code for the coronavirus spike protein to human cells that then grow it on their surface, training the immune system to be ready. The technology is now considered the leading Covid vaccination platform.
The vaccines were tested in trials of thousands of children. They were found to cause similar levels of mild side effects as in older age groups and triggered similar levels of antibodies.
Efficacy against infection was higher for Pfizer, with the company placing it at 80 percent, compared to Moderna's estimates of 51 percent for children aged six-months to two years old and 37 percent for those aged two to five years.
But the Pfizer figure is based on very few cases and is thus considered preliminary. It also takes three doses to achieve its protection, with the third shot given eight weeks after the second, which is given three weeks after the first.
Moderna's vaccine should provide strong protection against severe disease after two doses, given four weeks apart, and the company is studying adding a booster that would raise efficacy levels against mild disease.
However, Moderna's decision to go with a higher dose is associated with higher levels of fevers in reaction to the vaccine compared to Pfizer.
There are some 20 million children aged four years and under in the United States.
P.Stevenson--AMWN


