
-
 Ukraine accuses Hungary of taking 'hostage' bank staff carrying $40 mn
Ukraine accuses Hungary of taking 'hostage' bank staff carrying $40 mn
-
Aston Martin chief Newey says no quick fix to vibration problems

-
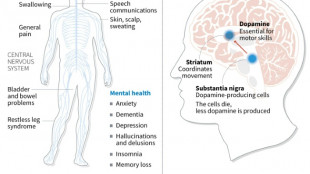 Japan approves stem-cell treatment for Parkinson's in world first
Japan approves stem-cell treatment for Parkinson's in world first
-
Heavy attacks hit Tehran as Israel says war in 'new phase'

-
 North Korea thrash Bangladesh in Women's Asian Cup warning
North Korea thrash Bangladesh in Women's Asian Cup warning
-
Hong Kong mogul Jimmy Lai will not appeal national security conviction: lawyer

-
 Eight dead, four missing in Brazil seniors home collapse
Eight dead, four missing in Brazil seniors home collapse
-
Paralympics brace for tense opening as Russia comes in from the cold

-
 Leclerc edges Hamilton to go fastest in first Australian GP practice
Leclerc edges Hamilton to go fastest in first Australian GP practice
-
Equities mostly drop as Mideast crisis rages, though oil dips

-
 Nepal counts votes after key post-uprising election
Nepal counts votes after key post-uprising election
-
Italy half-backs can make difference against England: ex-coach Mallett

-
 Scotland coach Townsend hails 'instinctive' France ahead of key Six Nations game
Scotland coach Townsend hails 'instinctive' France ahead of key Six Nations game
-
French starlet Seixas to take on Pogacar at Strade Bianche

-
 Brazil's Petrobras sees profit soar on record output
Brazil's Petrobras sees profit soar on record output
-
Arsenal, Chelsea aim to avoid FA Cup upsets

-
 US, Venezuela restore ties as Washington pushes for minerals access
US, Venezuela restore ties as Washington pushes for minerals access
-
Middle East war enters seventh day as Israel strikes Beirut

-
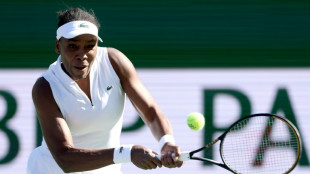 Qualifier Parry ends Venus's desert dream
Qualifier Parry ends Venus's desert dream
-
Iran missile barrage sparks explosions over Tel Aviv

-
 US says Venezuela to protect mining firms as diplomatic ties restored
US says Venezuela to protect mining firms as diplomatic ties restored
-
Trump honors Messi and MLS Cup champion Miami teammates

-
 Dismal Spurs can still avoid relegation vows Tudor
Dismal Spurs can still avoid relegation vows Tudor
-
Berger sets early pace at Arnold Palmer with 'unbelievable' 63

-
 Morocco part company with coach Regragui as World Cup looms
Morocco part company with coach Regragui as World Cup looms
-
Lens beat Lyon on penalties to reach French Cup semis

-
 El Salvador's Bukele holding dozens of political prisoners: rights group
El Salvador's Bukele holding dozens of political prisoners: rights group
-
With Iran war, US goes it alone like never before

-
 Spurs slip deeper into relegation trouble after loss to Palace
Spurs slip deeper into relegation trouble after loss to Palace
-
Pete Hegseth: Trump's Iran war attack dog

-
 Celtics' Tatum could make injury return on Friday
Celtics' Tatum could make injury return on Friday
-
'Enemy at home': Iranian authorities tighten grip as war rages

-
 Bethell set for 'hell of a career', says England captain Brook
Bethell set for 'hell of a career', says England captain Brook
-
France coach Galthie slams Scotland for 'smallest changing room in the world'

-
 Medvedev arrives in Indian Wells after being stranded in Dubai
Medvedev arrives in Indian Wells after being stranded in Dubai
-
Trump fires homeland security chief Kristi Noem

-
 Mideast war risks pulling more in as conflict boils over
Mideast war risks pulling more in as conflict boils over
-
Wales' James Botham 'sledged' by grandfather Ian Botham after Six Nations error

-
 India hero Samson eyes 'one more' big knock in T20 World Cup final
India hero Samson eyes 'one more' big knock in T20 World Cup final
-
Britney Spears detained on suspicion of driving while intoxicated

-
 Grooming makes Crufts debut as UK dog show widens offer
Grooming makes Crufts debut as UK dog show widens offer
-
Townsend insists Scots' focus solely on France not Six Nations title race

-
 UK sends more fighter jets to Gulf: PM
UK sends more fighter jets to Gulf: PM
-
EU to ban plant-based 'bacon' but veggie 'burgers' survive chop

-
 Leagues Cup to hold matches in Mexico for first time
Leagues Cup to hold matches in Mexico for first time
-
India reach T20 World Cup final after England fail in epic chase

-
 Conservative Anglicans press opposition to Church's first woman leader
Conservative Anglicans press opposition to Church's first woman leader
-
Sri Lanka takes control of Iranian ship fearing new US sub attack

-
 Iran players sing anthem and salute at Women's Asian Cup
Iran players sing anthem and salute at Women's Asian Cup
-
India beat England in high-scoring T20 World Cup semi-final


IGC Pharma Names IGC-AD1 Phase 2 Clinical Trial for Alzheimer's Agitation "CALMA" and Expands Recruitment Strategy
Innovative use of geofencing technology boosts enrollment at select sites
IGC Pharma, Inc. (NYSE American:IGC) ("IGC Pharma" or the "Company") announced today that its ongoing Phase 2 trial for agitation in Alzheimer's disease has been officially named CALMA (Calming Agitation in Alzheimer's).
The trial's new identity reflects its mission to address agitation, a syndrome affecting up to 76% of the 55 million Alzheimer's patients worldwide that also adversely impacts the quality of life for both patients and their caregivers.
To accelerate enrollment across all clinical sites, IGC Pharma has implemented an innovative recruitment campaign leveraging geofencing technology and digital outreach through social media platforms. The recruitment strategy was piloted at select trial sites, where it achieved a remarkable 200-300% increase in enrollment at a relatively low cost per lead. Building on this success, IGC Pharma is expanding the campaign to the other trial locations, with the goal of completing enrollment and the CALMA trial in the second half of 2025.
By combining advanced outreach tools with CALMA's clear focus, IGC aims to enhance engagement with patients and caregivers while advancing toward key milestones in Alzheimer's treatment development.
Ram Mukunda, CEO of IGC Pharma, commented, "Recruitment is one of the most significant challenges in Alzheimer's clinical trials, and our use of innovative geofencing technology, wherein we digitally target individuals living in a 15-25-mile radius of a clinical site, has delivered impressive results, achieving a significant outreach and enrollment at select sites. Scaling this strategy across all trial locations, in the USA and Canada, we expect will accelerate our progress toward completing the CALMA trial and strengthen our ability to engage with patients and caregivers. The strategy is especially powerful in recruiting members of underserved communities. By leveraging advanced digital outreach tools, we aim to enhance trial awareness, streamline recruitment, and advance IGC-AD1's development as a transformative therapy for Alzheimer's agitation. This milestone represents another step forward in creating value for both patients and our shareholders."
About CALMA
The CALMA trial is a multicenter, double-blind, randomized, placebo-controlled Phase 2 study involving 164 participants. It is designed to evaluate the safety and efficacy of IGC-AD1, a partial CB1 receptor agonist with anti-neuroinflammatory properties, in treating agitation - a syndrome that accelerates cognitive decline and increases hospitalization rates among Alzheimer's patients.
Individuals can learn more about the Calma trial here: Facebook page
About IGC Pharma (dba IGC):
IGC Pharma is an AI-powered, clinical-stage biotechnology company focused on developing innovative treatments for Alzheimer's disease and transforming patient care with fast-acting, safe, and effective solutions. Our portfolio includes the TGR family, including TGR-63, which targets amyloid plaques, a hallmark of Alzheimer's. The IGC-C and IGC-M platforms are advancing in preclinical studies, focusing on metabolic disorders, tau proteins, early plaque formation, and multiple disease hallmarks. Our lead therapeutic candidate, IGC-AD1, is a cannabinoid-based treatment currently in a Phase 2 trial for agitation in dementia associated with Alzheimer's (clinicaltrials.gov, NCT05543681). Interim data for IGC-AD1 demonstrated that it has the potential to transform patient care by offering faster-acting and more effective relief compared to traditional medications. Additionally, our AI models are designed to predict potential biomarkers for the early detection of Alzheimer's, optimize clinical trials, and predict receptor affinity, among others. With 28 patent filings and a commitment to innovation, IGC Pharma is dedicated to advancing pharmaceutical treatments and improving the lives of those affected by Alzheimer's and related conditions.
Forward-looking Statements
This press release contains forward-looking statements. These forward-looking statements are based largely on IGC Pharma's expectations and are subject to several risks and uncertainties, certain of which are beyond IGC Pharma's control. Actual results could differ materially from these forward-looking statements as a result of, among other factors, the Company's failure or inability to commercialize one or more of the Company's products or technologies, including the products or formulations described in this release, or failure to obtain regulatory approval for the products or formulations, where required, or government regulations affecting AI or the AI algorithms not working as intended or producing accurate predictions; general economic conditions that are less favorable than expected; the FDA's general position regarding cannabis- and hemp-based products; and other factors, many of which are discussed in IGC Pharma's U.S. Securities and Exchange Commission ("SEC") filings. IGC incorporates by reference its Annual Report on Form 10-K filed with the SEC on June 24, 2024, and on Form 10-Q filed with the SEC on August 7, 2024, as if fully incorporated and restated herein. Considering these risks and uncertainties, there can be no assurance that the forward-looking information contained in this release will occur.
Contact Information
Rosalyn Christian / Walter Frank
IMS Investor Relations
[email protected]
(203) 972-9200
SOURCE: IGC Pharma, Inc.
C.Garcia--AMWN



