
-
 South Korea outclass Iran in Asian Women's Cup opener
South Korea outclass Iran in Asian Women's Cup opener
-
Liverpool's Slot says his 'football heart' does not like set-piece trend

-
 Israel aims fresh attack at Tehran: latest developments in US-Iran war
Israel aims fresh attack at Tehran: latest developments in US-Iran war
-
Energy prices soar, stock markets slide on Iran war fallout

-
 'No indication' Iran nuclear installations hit: IAEA
'No indication' Iran nuclear installations hit: IAEA
-
Showdown looms between Tesla and German union

-
 Israel vows intensified attacks: latest developments in US-Iran war
Israel vows intensified attacks: latest developments in US-Iran war
-
France arrests activists blocking ship over alleged Russia uranium links

-
 Tech sovereignty and AI networks set to dominate mobile meet
Tech sovereignty and AI networks set to dominate mobile meet
-
Indian police clash with pro-Khamenei protesters in Kashmir

-
 Israel targets Hezbollah, Iran: latest developments in US-Iran war
Israel targets Hezbollah, Iran: latest developments in US-Iran war
-
Canada and India strike agreements on rare earth, uranium

-
 A rough guide to F1 rule changes for 2026
A rough guide to F1 rule changes for 2026
-
At least 25 killed at Pakistan's pro-Iran weekend protests

-
 Israel kills 31 in Lebanon, vows to expand strikes after Hezbollah fire
Israel kills 31 in Lebanon, vows to expand strikes after Hezbollah fire
-
Myanmar grants amnesty to over 7,000 convicted of 'terrorist group' support

-
 Riyadh's King Fahd stadium to host 2027 Asian Cup final
Riyadh's King Fahd stadium to host 2027 Asian Cup final
-
'Superman Sanju' toast of India after T20 World Cup heroics

-
 Travel chaos, but F1 season-opener in Australia 'ready to go'
Travel chaos, but F1 season-opener in Australia 'ready to go'
-
Lunar New Year heartache for Chinese team at Women's Asian Cup

-
 El Nino may return in 2026 and make planet even hotter
El Nino may return in 2026 and make planet even hotter
-
Somaliland's Israel deal could put Berbera port at risk

-
 Texas primaries launch midterm battle with Trump agenda at stake
Texas primaries launch midterm battle with Trump agenda at stake
-
How a Syrian refugee chef met Britain's King Charles

-
 Bangladesh tackle gender barriers to reach Women's Asian Cup
Bangladesh tackle gender barriers to reach Women's Asian Cup
-
Argentina's Milei says wants US 'strategic alliance' to be state policy

-
 'Sinners' wins top prize at Screen Actors Guild awards
'Sinners' wins top prize at Screen Actors Guild awards
-
New rules, same old suspects as F1 revs up for 2026 season

-
 World Cup tickets: Huge demand and sky-high prices
World Cup tickets: Huge demand and sky-high prices
-
List of key Actor Award winners

-
 Trump hunkers down after Iran strikes
Trump hunkers down after Iran strikes
-
China's leaders gather for key strategy session as challenges grow

-
 UK toughens asylum rules to discourage migration
UK toughens asylum rules to discourage migration
-
Israel hits Lebanon after Hezbollah fire, expanding Iran war

-
 CBS in turmoil as US media feels pressure under Trump
CBS in turmoil as US media feels pressure under Trump
-
Messi bags double as Miami battle back to down Orlando

-
 Greenland is 'open for business' -- kind of, says business leader
Greenland is 'open for business' -- kind of, says business leader
-
Canada's Carney to mend rift, boost trade as he meets India's Modi

-
 Crude soars, stocks drop after US strikes on Iran
Crude soars, stocks drop after US strikes on Iran
-
Iran war spreads across region as US, Israel suffer losses

-
 Miriam Margolyes tackles aging in Oscar-nominated short
Miriam Margolyes tackles aging in Oscar-nominated short
-
Recognition, not competition, for Oscar-nominated foreign filmmakers

-
 Israel, Hezbollah trade fire: latest developments in Iran war
Israel, Hezbollah trade fire: latest developments in Iran war
-
Israel strikes Tehran: latest developments in Iran war

-
 Trump vows to avenge first US deaths as Iran war intensifies
Trump vows to avenge first US deaths as Iran war intensifies
-
Habi Acquires Pulppo to Expand Leadership in Latin America's Residential Real Estate Market

-
 Who Is the Best Plastic Surgeon in Bellevue?
Who Is the Best Plastic Surgeon in Bellevue?
-
vMOX Merges with Advantage Communications Group

-
 Who Does the Cheapest Breast Augmentation in Florida?
Who Does the Cheapest Breast Augmentation in Florida?
-
MWC 2026: Amdocs Unveils CES26, an Agent-driven BSS-OSS-Network Suite, powered by the Amdocs aOS Cognitive Core

Tharimmune Announces Positive Results for Novel Oral Monoclonal Antibody TH023 Targeting Tumor Necrosis Factor-alpha
BRIDGEWATER, NJ / ACCESS Newswire / March 24, 2025 / Tharimmune, Inc. (Nasdaq:THAR) ("Tharimmune" or the "Company"), a clinical-stage biotechnology company focused on immunology and inflammation, today announced positive preclinical results for its novel oral antibody, TH023. In a murine model, a proprietary protease enzyme stabilized platform demonstrated successful delivery of infliximab, a tumor necrosis factor-alpha (TNF-α) inhibitor, in serum with concentrations detected being significantly higher than the standard serum trough concentration needed for antibody efficacy in immunology indications via injection (~3-5µg/ml). These findings represent a significant step towards developing a more convenient and potentially patient-preferred alternative to currently available infliximab treatments, which are administered via intravenous infusion or subcutaneous injection.
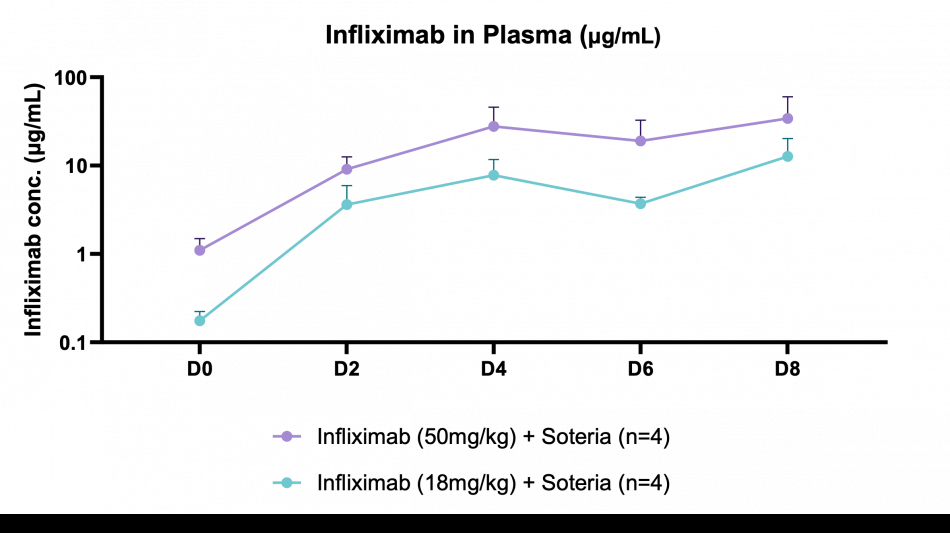
Key findings of the preclinical evaluation include demonstrating enzymatic protection of infliximab against human colon enzymes ex vivo using fresh fecal samples from healthy subjects utilizing the Soteria® platform, a proprietary formulation of natural amino acids (data not shown). Furthermore, successful delivery of TH023 in vivo into both local colonic tissue and systemic circulation was shown following intra-duodenal once-daily dosing for 1 week in a healthy mouse model at two doses of infliximab. This data shows the potential of the delivery platform to allow for both local delivery of the antibody precisely in the large intestinal tissue through enzymatic stabilization, as well as systemic circulation, which is an ideal pharmacokinetic (PK) profile for targeting both local gastrointestinal (GI) diseases such as inflammatory bowel disease (IBD) as well as systemic inflammatory diseases. The mechanism by which the antibody transcytosis occurs in the GI tract was shown to be a combination of passive, as well as mediated via the neonatal fragment crystallizable receptor (FcRn), highly expressed in distal intestinal epithelial cells enabling active transport.
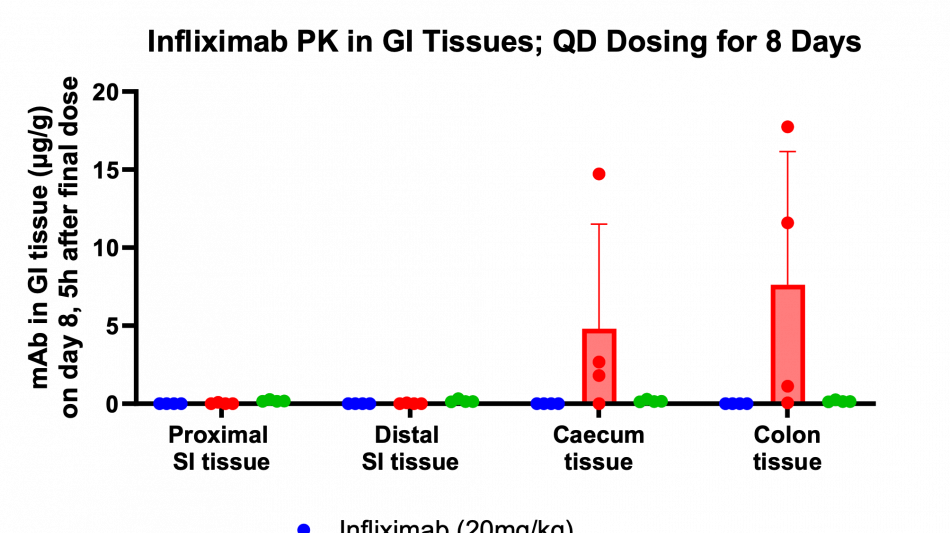
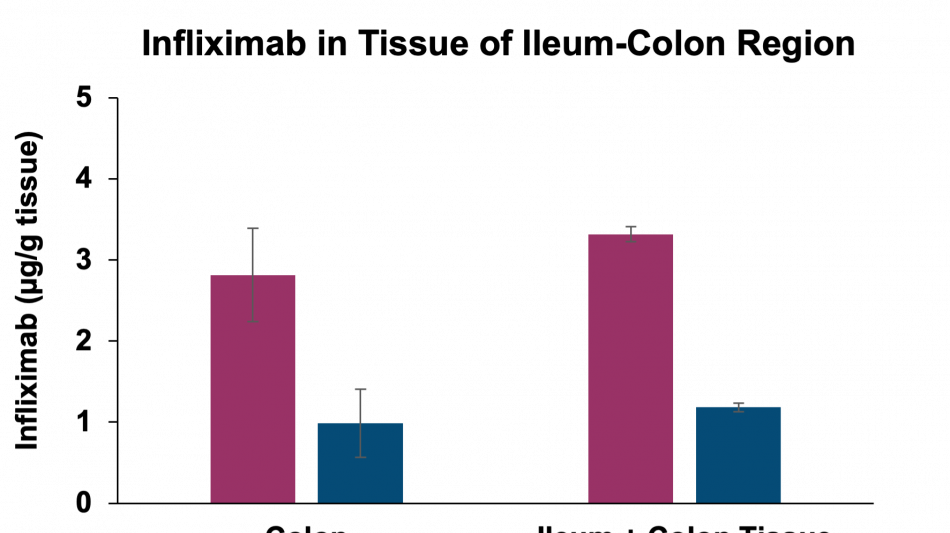
Additionally, the study demonstrated that tissue penetration of infliximab in combination with the enzyme stabilization platform was superior to a traditional permeation enhancer, sodium N-(8-[2-hydroxylbenzoyl] amino) caprylate (SNAC), which has been used to enhance the absorption of GLP-1 peptides, such as semaglutide. Utilization of SNAC to protect infliximab from enzymatic degradation or permeation enhancement did not result in tissue or serum concentrations suggesting standard off-the-shelf oral peptide delivery technologies are not suitable for oral delivery of antibodies. Two other standard permeation enhancer technologies tested (sodium caprate and labrasol) also showed unsitable results for oral delivery (data not shown), further supporting the Company's proprietary TH023 formulation.
The Company announced last year through a partnership with Intract Pharma, an exclusive license to INT-023 (now TH023), an oral anti-TNF-α monoclonal antibody. Tharimmune licensed global development and commercialization rights (outside of South Korea) to Intract Pharma's Soteria® and Phloral® delivery platform along with an existing supply agreement for infliximab to be used in the oral product development program. Traditionally administered through intravenous infusions, oral delivery of antibodies is challenging due to the complexity of navigating such large molecules through the GI tract. An oral route of administration holds potential to improve patient compliance and quality of life, while also reducing the burden on the healthcare system associated with long-term intravenous therapy.
"We are extremely encouraged by these results, which validate the potential of our partnership with Intract and the platform to deliver complex biologic molecules like infliximab orally," said Randy Milby, CEO of Tharimmune. "This represents a potential major milestone in our mission to develop more patient-friendly and accessible treatment options for chronic inflammatory diseases, addressing a multi-billion dollar market. These findings provide a strong foundation for further development into clinical trials."
Through the Company's existing partnership with Intract the data announced today enables for the targeted delivery of antibody therapeutics directly to the colon or small intestine. By leveraging Intract's platform, Tharimmune aims to enhance the effectiveness of TNF-α inhibitors such as infliximab through precision delivery that maximizes proteolytic stabilization and tissue permeation. This novel approach offers significant potential for directly addressing inflammatory conditions within the GI tract, including IBD as well as systemic inflammatory disorders where TNF-α plays a critical role in disease progression. Tharimmune plans to optimize the formulation and dosing regimen and prepare to conduct a first-in-human clinical trial with TH023 in the next 12 months.
Infliximab, a TNF-α inhibitor, is a widely used biologic for the treatment of several chronic inflammatory diseases, including Crohn's disease, ulcerative colitis, rheumatoid arthritis, ankylosing spondylitis, psoriatic arthritis, and plaque psoriasis. Globally, infliximab (including biosimilars) generated approximately $6.3 billion in sales in 2022, demonstrating the significant market for this therapy. However, current administration routes require frequent visits to healthcare facilities, which can be burdensome for patients and contribute to significant healthcare costs. Experts suggest the market for infliximab could rise to $9 billion within 10 years. An oral formulation of infliximab has the potential to improve patient convenience and compliance and eliminating the need for injections or infusions which could significantly improve the patient experience and potentially lead to better treatment adherence. Oral administration could reduce the need for clinic visits and specialized nursing care, potentially lowering overall treatment costs. A more convenient oral option could make infliximab accessible to a broader patient population, particularly in areas with limited access to infusion centers. By offering a differentiated, patient-preferred oral option, Tharimmune aims to capture a portion of the existing and growing infliximab market which represents a substantial commercial opportunity.
About Tharimmune, Inc.
Tharimmune is a clinical-stage biotechnology company developing a diverse portfolio of therapeutic candidates in immunology, inflammation and oncology. Its lead clinical asset, TH104, aims to suppress chronic pruritus associated with primary biliary cholangitis (PBC), a rare autoimmune liver disease with no known cure. The expanded pipeline includes TH023, an oral TNF-alpha inhibitor offering a new approach to treating autoimmune diseases. Tharimmune is also advancing early-stage multispecific biologics targeting unique epitopes against multiple solid tumors through its proprietary EpiClick™ Technology. The company has a license agreement with OmniAb, Inc. to access their antibody discovery technology for targeting specified disease markers. For more information, please visit: www.tharimmune.com.
Forward Looking Statements
Certain statements in this press release are forward-looking within the meaning of the Private Securities Litigation Reform Act of 1995. All statements, other than statements of historical facts, contained in this press release, including statements regarding the timing and design of Tharimmune's future Phase 2 trial, Tharimmune's strategy, future operations, future financial position, projected costs, prospects, plans and objectives of management, are forward-looking statements. The words "anticipate," "believe," "continue," "could," "depends," "estimate," "expect," "intend," "may," "ongoing," "plan," "potential," "predict," "project," "target," "should," "will," "would," and similar expressions are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words. The Company may not actually achieve the plans, intentions, or expectations disclosed in these forward-looking statements, and you should not place undue reliance on these forward-looking statements. Actual results or events could differ materially from the plans, intentions and expectations disclosed in these forward-looking statements. Factors that may cause such differences, include, but are not limited to, those discussed under Risk Factors set forth in our Annual Report on Form 10-K for the year ended December 31, 2023 and other periodic reports filed by the Company from time to time with the Securities and Exchange Commission. In addition, the forward-looking statements included in this press release represent the Company's views as of the date of this release. Subsequent events and developments may cause the Company's views to change; however, the Company does not undertake and specifically disclaims any obligation to update or revise any forward-looking statements to reflect new information, future events or circumstances or to reflect the occurrences of unanticipated events, except as may be required by applicable law. These forward-looking statements should not be relied upon as representing the Company's views as of any date subsequent to the date of this release.
Contacts:
Tharimmune, Inc.
[email protected]
Alliance Advisors IR
Tirth T. Patel
[email protected]
212-201-6614
SOURCE: Tharimmune Inc.
View the original press release on ACCESS Newswire
G.Stevens--AMWN
