
-
 South Africa 'embrace pressure' and favourites tag, says coach
South Africa 'embrace pressure' and favourites tag, says coach
-
Tel Aviv residents say ready to withstand more Iranian attacks

-
 Russia loses key ally leader as Putin slams Khamenei 'cynical' killing
Russia loses key ally leader as Putin slams Khamenei 'cynical' killing
-
AC Milan consolidate top-four credentials with win at Cremonese

-
 Flights of fancy at Bottega Veneta, atmospheric mood at Armani in Milan
Flights of fancy at Bottega Veneta, atmospheric mood at Armani in Milan
-
Guardiola calls for respect after Ramadan break is booed

-
 Afghanistan warns Iran war will impact whole region
Afghanistan warns Iran war will impact whole region
-
Iran launches fresh strikes across Gulf after vowing revenge for slain leader

-
 OPEC+ hikes oil production by more than expected following outbreak of Iran war
OPEC+ hikes oil production by more than expected following outbreak of Iran war
-
Goggia tightens grip on World Cup super-G with victory in Andorra

-
 Belgium seizes Russian 'shadow fleet' tanker
Belgium seizes Russian 'shadow fleet' tanker
-
Raza steers Zimbabwe to 153-7 against South Africa

-
 Kerr on target as Australia make winning start to Women's Asian Cup
Kerr on target as Australia make winning start to Women's Asian Cup
-
Marquez says 'unlucky' to retire from MotoGP season opener

-
 9 killed in pro-Iran protest at US consulate in Pakistan's Karachi
9 killed in pro-Iran protest at US consulate in Pakistan's Karachi
-
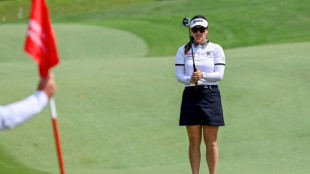
Green clinches Singapore title with help from caddie husband
-
 More flights cancelled as Iran conflict shuts Mideast hubs
More flights cancelled as Iran conflict shuts Mideast hubs
-
'One Battle After Another' wins top producer award before Oscars

-
 Iran vows revenge for slain supreme leader despite Trump threat
Iran vows revenge for slain supreme leader despite Trump threat
-
Flights of fancy at Bottega Veneta with shimmering, tactile collection

-
 World Cup marks 100-day countdown amid political upheaval
World Cup marks 100-day countdown amid political upheaval
-
Bezzecchi wins MotoGP opener as Marquez retires

-
 Pro-Iran protesters try to storm US missions in Pakistan, Iraq
Pro-Iran protesters try to storm US missions in Pakistan, Iraq
-
8 killed in pro-Iran protest at US consulate in Pakistan's Karachi

-
 Latest developments after US, Israeli strikes kill Iran's Khamenei
Latest developments after US, Israeli strikes kill Iran's Khamenei
-
Before dawn, ancient drum rite wakes Istanbul faithful to fast

-
 Music, mourning as Iran's Khamenei is killed
Music, mourning as Iran's Khamenei is killed
-
Pakistan cricket's lack of T20 evolution exposed by World Cup exit

-
 Cobolli downs Tiafoe to claim Mexican Open
Cobolli downs Tiafoe to claim Mexican Open
-
Takele defends Tokyo Marathon title after sprint finish

-
 Hollywood's finest gather for guild's Actor Awards
Hollywood's finest gather for guild's Actor Awards
-
Iran prepare for Women's Asian Cup as bombs drop on homeland

-
 Doncic shines as Lakers cruise past depleted Warriors
Doncic shines as Lakers cruise past depleted Warriors
-
3D tool Unreal Engine makes real impact in creative industries

-
 OPEC+ mulls oil production increase in shadow of war
OPEC+ mulls oil production increase in shadow of war
-
Putin, Russia's eternal leader defined by war and power

-
 Explosion, gunfire as Afghan forces shoot at aircraft over Kabul
Explosion, gunfire as Afghan forces shoot at aircraft over Kabul
-
Iranians across North America rally for -- and against -- strikes

-
 Shakespeare would have shunned streaming, 'Hamnet' team says
Shakespeare would have shunned streaming, 'Hamnet' team says
-
Will Oscars be 17th time lucky for songwriter Diane Warren?

-
 Sympathy for the bedeviled: the likable conspiracy theorist of 'Bugonia'
Sympathy for the bedeviled: the likable conspiracy theorist of 'Bugonia'
-
Texas port humming as Trump ramps up Venezuela oil

-
 76ers' center Embiid to miss at least three games with oblique strain
76ers' center Embiid to miss at least three games with oblique strain
-
US, Israel defend strikes at UN as Iran alleges 'war crime'

-
 Brumbies' 'mental resolve' keeps them unbeaten in Super Rugby
Brumbies' 'mental resolve' keeps them unbeaten in Super Rugby
-
Iran attacks rock Dubai's Palm, Burj Al Arab, airport

-
 Sterling Metals Discovers Cu-Mo Porphyry Stock 1.5km from MEPS Discovery Zone Confirming Scale of Porphyry Copper System
Sterling Metals Discovers Cu-Mo Porphyry Stock 1.5km from MEPS Discovery Zone Confirming Scale of Porphyry Copper System
-
JP Anderson Signs Landmark MOU with Vaama Village to Advance Rare Earth Mineral Development in Bonthe District

-
 Iran leader Khamenei killed in massive US and Israeli attack, Trump says
Iran leader Khamenei killed in massive US and Israeli attack, Trump says
-
UK pop-soul star Olivia Dean sweeps Brit Awards


enVVeno Medical Reports First Quarter 2025 Financial Results and Reiterates Progress Toward VenoValve FDA Decision Expected in 2H24
Cash Burn of $4.0 million in Q1 remains in line with projected quarterly range
Cash and investments on hand are sufficient to fund operations beyond the anticipated FDA decision of VenoValve and the initiation of the enVVe pivotal trial
FDA decision on PMA application for the VenoValve expected in the second half of 2025
On track for enVVe IDE application submission in Q3 of 2025, pending GLP study results
IRVINE, CA / ACCESS Newswire / May 1, 2025 / enVVeno Medical Corporation (NASDAQ:NVNO) ("enVVeno" or the "Company"), a company setting new standards of care for the treatment of deep venous disease, today reported financial results for the first quarter 2025.
Robert Berman, enVVeno Medical's Chief Executive Officer, commented "In the first quarter, we continued to present our compelling 1-year data from the U.S. pivotal trial at leading global, scientific conferences to socialize and engage directly with leading vascular surgeons as we lay the foundation for the potential phased market entry of the VenoValve, pending FDA approval. We have also maintained a strong financial position - an important advantage in the current market conditions. We remain confident that 2025 will be a pivotal year as we prepare for our transition from a development-stage company to a commercial enterprise."
Summary of Financial Results for the First Quarter 2025
The Company ended the quarter with $38.9 million in cash and investments. Based on management's current expectations, this capital has the potential to fund the Company through the anticipated FDA decision for VenoValve, the initiation of commercialization preparations for VenoValve and the commencement of the enVVe pivotal study.
Cash burn for the quarter was $4.0 million, consistent with the Company's projected cash burn rate of approximately $4-5 million per quarter. The Company anticipates that its cash burn rate will increase from current levels once commercialization of the VenoValve begins.
The Company reported net losses of $4.5 million and $5.0 million for the three months ended March 31, 2025 and 2024, respectively, representing a decrease in net loss of $0.5 million, primarily resulting from a decrease in operating expenses.
Clinical Program Highlights
VenoValve®: Surgical Replacement Venous Valve
VenoValve PMA application seeking U.S. Food and Drug Administration (FDA) approval submitted; Decision expected in H2 2025.
The Company estimates that there are approximately 2.5 million potential new patients each year in the U.S. that could be candidates for the SAVVE procedure, including approximately 1.5 million with active venous ulcers.
enVVe®: Non-Surgical Transcatheter Based Replacement Venous Valve
Successfully completed final wave for the shorter-term subjects in 6-month pre-clinical GLP study. The follow-up period, which began with the first wave of implants, is ongoing as scheduled.
The GLP study should be the final step necessary before filing the Investigational Device Exemption (IDE) seeking FDA approval to start the enVVe pivotal study.
The Company expects to be in a position to file for IDE approval for the enVVe pivotal study mid-2025.
About enVVeno Medical Corporation
enVVeno Medical (NASDAQ:NVNO) is an Irvine, California-based, late clinical-stage medical device Company focused on the advancement of innovative bioprosthetic (tissue-based) solutions to improve the standard of care for the treatment of deep venous disease. The Company's lead product, the VenoValve®, is a first-in-class surgical replacement venous valve being developed for the treatment of deep venous Chronic Venous Insufficiency (CVI). The Company is also developing a non-surgical, transcatheter based replacement venous valve for the treatment of deep venous CVI called enVVe®. CVI occurs when valves inside of the veins of the leg become damaged, resulting in the backwards flow of blood (reflux), blood pooling in the lower leg, increased pressure in the veins of the leg (venous hypertension) and in severe cases, venous ulcers that are difficult to heal and become chronic. Both the VenoValve and enVVe are designed to act as one-way valves, to help assist in propelling blood up the leg, and back to the heart and lungs. The Company has submitted a pre-market authorization (PMA) application for the VenoValve to the FDA, with a decision anticipated in the second half of 2025 and is currently performing the final testing necessary to seek approval from the FDA for the enVVe pivotal trial.
Cautionary Note on Forward-Looking Statements
This press release and any statements of stockholders, directors, employees, representatives and partners of enVVeno Medical Corporation (the "Company") related thereto contain, or may contain, among other things, certain "forward-looking statements" within the meaning of the Private Securities Litigation Reform Act of 1995. Such forward-looking statements involve significant risks and uncertainties. Such statements may include, without limitation, statements identified by words such as "projects," "may," "will," "could," "would," "should," "believes," "expects," "anticipates," "estimates," "intends," "plans," "potential" or similar expressions. These statements are based upon the current beliefs and expectations of the Company's management and are subject to significant risks and uncertainties, including those detailed in the Company's filings with the Securities and Exchange Commission. Actual results and timing (may differ significantly from those set forth or implied in the forward-looking statements. Forward-looking statements involve certain risks and uncertainties that are subject to change based on various factors (many of which are beyond the Company's control). The Company undertakes no obligation to publicly update any forward-looking statements, whether as a result of new information, future presentations or otherwise, except as required by applicable law.
INVESTOR CONTACT:
Jenene Thomas, JTC Team, LLC
[email protected]
(908) 824-0775
MEDIA CONTACT:
Glenn Silver, FINN Partners
[email protected]
(973) 818-8198
SOURCE: enVVeno Medical Corporation
View the original press release on ACCESS Newswire
P.Silva--AMWN


