
-
 OPEC+ hikes oil production by more than expected following outbreak of Iran war
OPEC+ hikes oil production by more than expected following outbreak of Iran war
-
Goggia tightens grip on World Cup super-G with victory in Andorra

-
 Belgium seizes Russian 'shadow fleet' tanker
Belgium seizes Russian 'shadow fleet' tanker
-
Raza steers Zimbabwe to 153-7 against South Africa

-
 Kerr on target as Australia make winning start to Women's Asian Cup
Kerr on target as Australia make winning start to Women's Asian Cup
-
Marquez says 'unlucky' to retire from MotoGP season opener

-
 9 killed in pro-Iran protest at US consulate in Pakistan's Karachi
9 killed in pro-Iran protest at US consulate in Pakistan's Karachi
-
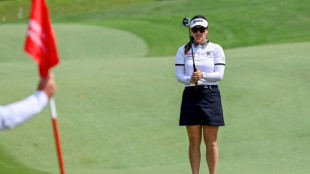
Green clinches Singapore title with help from caddie husband
-
 More flights cancelled as Iran conflict shuts Mideast hubs
More flights cancelled as Iran conflict shuts Mideast hubs
-
'One Battle After Another' wins top producer award before Oscars

-
 Iran vows revenge for slain supreme leader despite Trump threat
Iran vows revenge for slain supreme leader despite Trump threat
-
Flights of fancy at Bottega Veneta with shimmering, tactile collection

-
 World Cup marks 100-day countdown amid political upheaval
World Cup marks 100-day countdown amid political upheaval
-
Bezzecchi wins MotoGP opener as Marquez retires

-
 Pro-Iran protesters try to storm US missions in Pakistan, Iraq
Pro-Iran protesters try to storm US missions in Pakistan, Iraq
-
8 killed in pro-Iran protest at US consulate in Pakistan's Karachi

-
 Latest developments after US, Israeli strikes kill Iran's Khamenei
Latest developments after US, Israeli strikes kill Iran's Khamenei
-
Before dawn, ancient drum rite wakes Istanbul faithful to fast

-
 Music, mourning as Iran's Khamenei is killed
Music, mourning as Iran's Khamenei is killed
-
Pakistan cricket's lack of T20 evolution exposed by World Cup exit

-
 Cobolli downs Tiafoe to claim Mexican Open
Cobolli downs Tiafoe to claim Mexican Open
-
Takele defends Tokyo Marathon title after sprint finish

-
 Hollywood's finest gather for guild's Actor Awards
Hollywood's finest gather for guild's Actor Awards
-
Iran prepare for Women's Asian Cup as bombs drop on homeland

-
 Doncic shines as Lakers cruise past depleted Warriors
Doncic shines as Lakers cruise past depleted Warriors
-
3D tool Unreal Engine makes real impact in creative industries

-
 OPEC+ mulls oil production increase in shadow of war
OPEC+ mulls oil production increase in shadow of war
-
Putin, Russia's eternal leader defined by war and power

-
 Explosion, gunfire as Afghan forces shoot at aircraft over Kabul
Explosion, gunfire as Afghan forces shoot at aircraft over Kabul
-
Iranians across North America rally for -- and against -- strikes

-
 Shakespeare would have shunned streaming, 'Hamnet' team says
Shakespeare would have shunned streaming, 'Hamnet' team says
-
Will Oscars be 17th time lucky for songwriter Diane Warren?

-
 Sympathy for the bedeviled: the likable conspiracy theorist of 'Bugonia'
Sympathy for the bedeviled: the likable conspiracy theorist of 'Bugonia'
-
Texas port humming as Trump ramps up Venezuela oil

-
 76ers' center Embiid to miss at least three games with oblique strain
76ers' center Embiid to miss at least three games with oblique strain
-
US, Israel defend strikes at UN as Iran alleges 'war crime'

-
 Brumbies' 'mental resolve' keeps them unbeaten in Super Rugby
Brumbies' 'mental resolve' keeps them unbeaten in Super Rugby
-
Iran attacks rock Dubai's Palm, Burj Al Arab, airport

-
 JP Anderson Signs Landmark MOU with Vaama Village to Advance Rare Earth Mineral Development in Bonthe District
JP Anderson Signs Landmark MOU with Vaama Village to Advance Rare Earth Mineral Development in Bonthe District
-
Iran leader Khamenei killed in massive US and Israeli attack, Trump says

-
 UK pop-soul star Olivia Dean sweeps Brit Awards
UK pop-soul star Olivia Dean sweeps Brit Awards
-
Iranians across North America take to the streets for - and against - strikes

-
 'Turning point' as Crusaders notch first Super Rugby win
'Turning point' as Crusaders notch first Super Rugby win
-
White House releases photos of Trump, Vance during Iran ops

-
 PSG win to extend lead over Lens at top of Ligue 1
PSG win to extend lead over Lens at top of Ligue 1
-
Barca's Yamal nets hat-trick in Villarreal romp, Atletico go third

-
 Trump says Khamenei is dead after Israel, US attack Iran
Trump says Khamenei is dead after Israel, US attack Iran
-
Iran's Khamenei: ruthless revolutionary atop Islamic republic

-
 Inter continue Scudetto march after Champions League humbling
Inter continue Scudetto march after Champions League humbling
-
Questions cloud Trump's case for war against Iran


Tharimmune Presents Positive Clinical Data Highlighting TH104 Metabolic Profile and Advances Program for Prophylaxis Against Ultrapotent Opioid Exposure Following FDA Feedback
BRIDGEWATER, NEW JERSEY / ACCESS Newswire / May 6, 2025 / Tharimmune, Inc., (Nasdaq:THAR) ("Tharimmune" or the "Company") a clinical-stage biopharmaceutical company focused on developing innovative therapeutics in inflammation & immunology released pharmacokinetic and metabolism data from its Phase 1 study of TH104, a buccal film formulation of nalmefene, in healthy subjects. Tharimmune recently highlighted the advancement of TH104 for the proposed indication of temporary prophylaxis against respiratory and/or central nervous system depression in military personnel and chemical incident responders exposed to high-potency opioids, following recent positive feedback from the U.S. Food and Drug Administration (FDA).
The Phase 1 study presented this week at Digestive Disease Week taking place in San Diego, California evaluated the pharmacokinetics and metabolism of a single dose of TH104 (16 mg buccal film) compared to an intravenous (IV) dose of nalmefene injection (1 mg) in healthy volunteers. The study successfully demonstrated that buccal administration of TH104 achieves systemic exposure to nalmefene while offering a distinct metabolic profile compared to IV administration.
Phases of drug metabolism (such as phase 1 and phase 2) denote the "breaking down" and "tagging" of drugs, generally into molecules known as metabolites, by the liver to prepare them for removal. The key difference with drugs taken by mouth is the "first-pass effect," where the drug goes through the liver's processing immediately after being absorbed from the gut, before the drug circulates throughout the body. Drugs injected into the bloodstream bypass this initial liver processing generally leading to more drug circulating in the body before being metabolized.
Key findings from the pharmacokinetic study presented at the Digestive Disease Week 2025 included:
Buccal administration of TH104 resulted in slower absorption compared to IV administration
The ratio of the area under the curve (AUC) of nalmefene glucuronide, a metabolite, compared to nalmefene was significantly higher for TH104 versus IV administration
Pharmacokinetics of nalmefene sulfate and nornalmefene, 2 other metabolites, were significantly delayed with TH104 compared to IV administration
Both formulations showed early phase 2 metabolism (glucuronidation), but importantly, TH104 demonstrated delayed phase 1 metabolism, which is mainly catalyzed by enzymes such as cytochrome P450 oxidases in the liver
By attenuating the burden on hepatic metabolic pathways, TH104 may represent a novel therapeutic candidate for individuals with impaired liver function. The altered pharmacokinetic profile, particularly the delayed onset of phase 1 metabolism observed with buccal administration may confer a potential advantage in populations with impaired hepatic function, and may be important in patients with primary biliary cholangitis (PBC). PBC is a rare cholestatic liver disease frequently associated with intractable pruritus. Tharimmune is also advancing TH104 as a therapeutic agent for the management of moderate-to-severe chronic pruritus in PBC patients.
"The compelling pharmacokinetic data from our Phase 1 study not only supports the continued development of TH104 for symptomatic pruritus in liver disease by highlighting a potentially liver-friendly metabolic profile but importantly provides a crucial foundation for our accelerated development pathway in the opioid prophylaxis setting," said Randy Milby, CEO of Tharimmune. "The positive feedback from the FDA, namely a 505(b)(2) submission without the need for additional clinical trials for the prophylaxis indication is a transformative milestone for Tharimmune and positions TH104 as our lead program to address a critical unmet need for military personnel and first responders facing the threat of high-potency opioid exposure."
The Phase 1 results in healthy subjects also demonstrated comparable safety and tolerability between TH104 and IV nalmefene, with only mild adverse events reported. Building on the favorable pharmacokinetic and safety profile, Tharimmune is advancing TH104 as a critical medical countermeasure product. Following positive feedback from the FDA, Tharimmune is pursuing a 505(b)(2) regulatory pathway for TH104 for the temporary prophylaxis of respiratory and/or CNS depression in military personnel and chemical incident responders who may encounter environments contaminated with high-potency opioids, including weaponized fentanyl and its analogues.
The FDA has indicated that no additional clinical trials appear to be necessary prior to the submission of a New Drug Application (NDA) for this indication, allowing Tharimmune to leverage existing safety and efficacy data for nalmefene along with the pharmacokinetic data generated with the TH104 buccal film. This expedited pathway underscores the urgent need for such a prophylactic solution, particularly for chemical incident responders operating in high-risk environments where exposure to highly potent opioids is a potential threat to national security. The buccal film formulation offers a distinct advantage for rapid and convenient administration, even when personnel are wearing protective gear.
About Tharimmune, Inc.
Tharimmune is a clinical-stage biotechnology company developing a diverse portfolio of therapeutic candidates in immunology, inflammation and oncology. Its lead clinical asset, TH104, is being developed for a specific indication via a 505(b)2 pathway for respiratory and/or nervous system depression in military personnel and chemical incident responders who may encounter environments contaminated with high-potency opioids. The expanded pipeline includes other indications for TH104, such as chronic pruritus in primary biliary cholangitis and TH023, a new approach to treating autoimmune diseases along with an early-stage multispecific biologic platform targeting unique epitopes against multiple solid tumors through its proprietary EpiClick™ Technology. The Company has a license agreement with OmniAb, Inc. to access their antibody discovery technology for targeting specified disease markers. Tharimmune continues to position itself as a leader in patient-centered innovation while working to deliver long-term value for shareholders. For more information, visit: www.tharimmune.com.
Forward Looking Statements
Certain statements in this press release are forward-looking within the meaning of the Private Securities Litigation Reform Act of 1995. All statements, other than statements of historical facts, contained in this press release, including statements regarding the timing and design of Tharimmune's future Phase 2 trial, Tharimmune's strategy, future operations, future financial position, projected costs, prospects, plans and objectives of management, are forward-looking statements. The words "anticipate," "believe," "continue," "could," "depends," "estimate," "expect," "intend," "may," "ongoing," "plan," "potential," "predict," "project," "target," "should," "will," "would," and similar expressions are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words. The Company may not actually achieve the plans, intentions, or expectations disclosed in these forward-looking statements, and you should not place undue reliance on these forward-looking statements. Actual results or events could differ materially from the plans, intentions and expectations disclosed in these forward-looking statements. Factors that may cause such differences, include, but are not limited to, those discussed under Risk Factors set forth in our Annual Report on Form 10-K for the year ended December 31, 2024 and other periodic reports filed by the Company from time to time with the Securities and Exchange Commission. In addition, the forward-looking statements included in this press release represent the Company's views as of the date of this release. Subsequent events and developments may cause the Company's views to change; however, the Company does not undertake and specifically disclaims any obligation to update or revise any forward-looking statements to reflect new information, future events or circumstances or to reflect the occurrences of unanticipated events, except as may be required by applicable law. These forward-looking statements should not be relied upon as representing the Company's views as of any date subsequent to the date of this release.
Contacts:
Tharimmune, Inc.
[email protected]
Alliance Advisors IR
Tirth T. Patel
[email protected]
212-201-6614
SOURCE: Tharimmune Inc.
View the original press release on ACCESS Newswire
M.A.Colin--AMWN


