
-
 Pakistan 'have underperformed' says Agha after T20 World Cup exit
Pakistan 'have underperformed' says Agha after T20 World Cup exit
-
Under-strength Toulouse overpower Montauban in Top 14

-
 Vietnam AI law takes effect, first in Southeast Asia
Vietnam AI law takes effect, first in Southeast Asia
-
Brazil's Lula visits flood zone as death toll from landslides hits 70

-
 New Zealand into T20 World Cup semis as Sri Lanka avoid big Pakistan loss
New Zealand into T20 World Cup semis as Sri Lanka avoid big Pakistan loss
-
Medvedev wins Dubai title as Griekspoor withdraws

-
 First Yamal hat-trick helps Liga leaders Barcelona beat Villarreal
First Yamal hat-trick helps Liga leaders Barcelona beat Villarreal
-
Liverpool hit five past West Ham, Haaland-less City face Leeds test

-
 Van der Poel romps to cobbled classic win
Van der Poel romps to cobbled classic win
-
Republicans back Trump, Democrats attack 'illegal' Iran war

-
 Madonna is surprise attraction at Dolce & Gabbana Milan show
Madonna is surprise attraction at Dolce & Gabbana Milan show
-
Farhan keeps Pakistan hopes alive as they post 212-8 against Sri Lanka

-
 Afghanistan says civilians killed in Pakistan air strikes
Afghanistan says civilians killed in Pakistan air strikes
-
Tug of war: how US presidents battle Congress for military powers

-
 Residents flee as Iran missiles stun peaceful Gulf cities
Residents flee as Iran missiles stun peaceful Gulf cities
-
Streets empty and shops close as US strikes confirm Iranian fears

-
 Israelis shelter underground as Iran fires missiles
Israelis shelter underground as Iran fires missiles
-
Bournemouth held by Sunderland in blow to European bid

-
 VAR expanded to include second bookings and corners for World Cup
VAR expanded to include second bookings and corners for World Cup
-
Iranians in Istanbul jittery but jubilant at US, Israeli strikes

-
 Congo-Brazzaville president vows to keep power as campaign kicks off
Congo-Brazzaville president vows to keep power as campaign kicks off
-
US, Israel launch strikes on Iran, Tehran hits back across region

-
 Germany's Aicher wins women's super-G in Soldeu
Germany's Aicher wins women's super-G in Soldeu
-
Fight against terror: Trump threatens Tehran's mullahs

-
 US and Israel launch strikes on Iran, explosions reported across region
US and Israel launch strikes on Iran, explosions reported across region
-
Iran's Khamenei: ruthless revolutionary at apex of Islamic republic

-
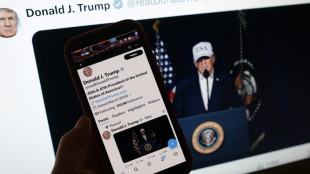 In Iran attack, Trump seeks what he foreswore -- regime change
In Iran attack, Trump seeks what he foreswore -- regime change
-
Climate change forces facelift for Michelangelo masterpiece

-
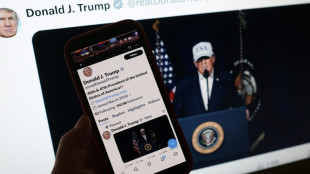 Trump says US aims to destroy Iran's military, topple government
Trump says US aims to destroy Iran's military, topple government
-
Acosta wins season-opening MotoGP sprint after Marquez penalty

-
 US and Israel launch strikes against Iran
US and Israel launch strikes against Iran
-
Afghanistan says Pakistan fighter jet down as cross-border strikes flare

-
 Kerr says only '85 percent' fit for Women's Asian Cup
Kerr says only '85 percent' fit for Women's Asian Cup
-
Messi's Inter Miami to visit White House: US media

-
 Thunder beat Nuggets in overtime on Gilgeous-Alexander's return
Thunder beat Nuggets in overtime on Gilgeous-Alexander's return
-
'It's surreal': Zimbabwe superfans revel in unexpected ride to India

-
 New 'Wuthering Heights' film unleashes fresh wave of Bronte-mania
New 'Wuthering Heights' film unleashes fresh wave of Bronte-mania
-
US backs Pakistan's 'right to defend itself' after strikes on Afghanistan

-
 Bezzecchi beats Marquez to pole at season-opening Thailand MotoGP
Bezzecchi beats Marquez to pole at season-opening Thailand MotoGP
-
OpenAI strikes Pentagon deal with 'safeguards' as Trump dumps Anthropic

-
 Oscar-nominated 'F1' sound engineers recreate roar of racetrack
Oscar-nominated 'F1' sound engineers recreate roar of racetrack
-
15 dead as cash-packed military plane crashes in Bolivia

-
 Costa Rica's Grynspan pledges reform in bid for UN chief job
Costa Rica's Grynspan pledges reform in bid for UN chief job
-
Former All Black Bridge hailed for influence at Western Force

-
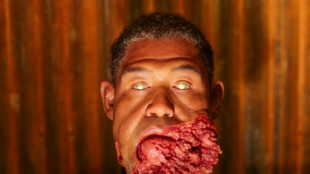 'Sinners' vampires inspired by animals, says Oscar hopeful makeup artist
'Sinners' vampires inspired by animals, says Oscar hopeful makeup artist
-
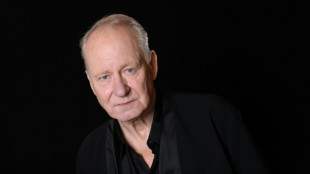
For Oscar nominee Stellan Skarsgard, good cinema is like slow food
-
 'Brilliant industry' sees Reds down Highlanders in Super Rugby
'Brilliant industry' sees Reds down Highlanders in Super Rugby
-
Neil Sedaka, US singer and songwriter, dies age 86
-
 New to The Street to Broadcast Executive Leadership Interviews Featuring Medicus Pharma Ltd. (NASDAQ:MDCX), CitroTech (NYSE:CITR), Vivos Therapeutics, Inc. (NASDAQ:VVOS), and Virtuix Holdings ($VTIX) on Bloomberg Television Tonight at 6:30 PM EST
New to The Street to Broadcast Executive Leadership Interviews Featuring Medicus Pharma Ltd. (NASDAQ:MDCX), CitroTech (NYSE:CITR), Vivos Therapeutics, Inc. (NASDAQ:VVOS), and Virtuix Holdings ($VTIX) on Bloomberg Television Tonight at 6:30 PM EST
-
Understanding Common Dental Issues That Develop Over Time in Richardson, TX


Positive Interim 2-Year Data from enVVeno Medical's VenoValve Pivotal Study to be Presented Today at the Society for Vascular Surgery (SVS) 2025 Vascular Annual Meeting
Interim two-year follow-up data from 42 subjects in the VenoValve pivotal trial show sustained clinical improvement and continued patient benefit at 24 months compared to baseline
83% of subjects maintained a clinically meaningful benefit with a 3 or more point improvement in Revised Venous Clinical Severity Score (rVCSS)
9.1 point average rVCSS improvement among the clinically meaningful benefit cohort
Subjects experienced a median 74% improvement in leg pain
Interim follow-up data indicate sustained improvements across all venous-specific quality-of-life (QoL) indicators
A decision from the U.S. Food and Drug Administration (FDA) on the VenoValve is anticipated in the second half of 2025
Company to host live webcast with presenting Principal Investigator, today, June 6th at 11:20 AM ET / 10:20 AM CT - Access the Webcast Here
IRVINE, CA / ACCESS Newswire / June 6, 2025 / enVVeno Medical Corporation (Nasdaq:NVNO) ("enVVeno" or the "Company"), a company setting new standards of care for the treatment of deep venous disease, today announced that interim two-year follow-up data on 42 subjects from the 75 person VenoValve U.S. pivotal trial will be presented today by lead enroller Dr. Cassius Iyad Ochoa Chaar, at the Society for Vascular Surgery (SVS) 2025 Vascular Annual Meeting (VAM25) being held June 4-7, 2025 in New Orleans, LA. Additionally, the Company announced it will host a live webcast to discuss these interim results today, Friday, June 6th at 11:20 AM ET / 10:20 AM CT (details below).
Key interim two-year follow-up data being presented at VAM25 include:
83.3% of subjects (n=35/42) maintained a clinically meaningful benefit, defined as an improvement of 3 or more points in the revised Venous Clinical Severity Score (rVCSS).
9.1 point average rVCSS improvement among the responder cohort.
A median 74% improvement in leg pain, as measured by the Visual Analog Scale (VAS).
Wound healing outcomes in 17 subjects with 25 ulcers showed that 60% of ulcers healed completely, 24% decreased in size, and 16% increased in size.
Patient-reported outcomes also demonstrated sustained improvements across all venous specific QoL indicators (VEINES-QoL/Sym).
Among the subjects (n=30), a 100% valve patency rate.
All values were calculated comparing each patient's baseline levels to the reported values at the patient's 24-month visit. The Revised Venous Clinical Severity Score (rVCSS) is a clinically validated scoring system used to track the progression or regression of venous diseases.
"These interim two-year follow-up data demonstrate substantial and sustained improvement across all effectiveness endpoints at two years. This is extremely encouraging, especially when you consider that all the patients enrolled in the study had severe CVI and failed all other treatment options," said Robert Berman, enVVeno Medical's Chief Executive Officer. "Despite attempts over many decades, nobody has been able to create an effective treatment for severe CVI caused by malfunctioning valves in the deep veins of the leg. The VenoValve has the potential to change the treatment paradigm for deep venous CVI, both for the millions of patients suffering from severe CVI and the thousands of vascular surgeons who have been waiting for an effective treatment option."
Dr. Cassius Iyad Ochoa Chaar, who is the Associate Professor of Surgery, Division of Vascular Surgery and Endovascular Therapy, Yale School of Medicine, will present the abstract titled, "Patients with Deep Venous Reflux Continue to Experience Clinical Improvement 2-year after Implantation of the VenoValve" today at VAM 2025.
The VenoValve is a potential first-in-class, surgical replacement venous valve for patients with severe deep venous CVI. The Company estimates that there are approximately 2.5 million potential new patients each year in the U.S. that could be candidates for the VenoValve. The Company has submitted a pre-market authorization (PMA) application for the VenoValve to the U.S. Food and Drug Administration (FDA), with a decision anticipated in the second half of 2025.
Webcast Details
The Company will host a webcast presentation to discuss the results for investors, analysts and other interested parties today, June 6, 2025, at 11:20 AM ET / 10:20 AM CT. Joining enVVeno management for the event will be Dr. Chaar. The live webcast will be accessible on the Events page of the enVVeno website, envveno.com, and will be archived for 90 days.
About CVI
Severe, deep venous Chronic Venous Insufficiency (CVI) is a debilitating disease that is most often caused by blood clots (deep vein thromboses or DVTs) in the deep veins of the leg. When valves inside of the veins of the leg fail, blood flows in the wrong direction and pools in the lower leg, causing pressure within the veins of the leg to increase (venous hypertension). Symptoms of severe CVI include leg swelling, pain, edema, and in the most severe cases, recurrent open sores known as venous ulcers. The disease can severely impact everyday functions such as sleeping, bathing, dressing, and walking, and is known to result in high rates of depression and anxiety. There are currently no effective treatments for severe CVI of the deep vein system caused by valvular incompetence. Estimates indicate that CVI costs the U.S. healthcare system in excess of $4 billion each year.
About enVVeno Medical Corporation
enVVeno Medical (NASDAQ:NVNO) is an Irvine, California-based, late clinical-stage medical device Company focused on the advancement of innovative bioprosthetic (tissue-based) solutions to improve the standard of care for the treatment of deep venous disease. The Company's lead product, the VenoValve®, is a first-in-class surgical replacement venous valve being developed for the treatment of deep venous Chronic Venous Insufficiency (CVI). The Company is also developing a non-surgical, transcatheter based replacement venous valve for the treatment of deep venous CVI called enVVe®. Both the VenoValve and enVVe are designed to act as one-way valves, to help assist in propelling blood up the leg, and back to the heart and lungs. The VenoValve is currently being evaluated in the VenoValve U.S. pivotal study and the Company is currently performing the final testing necessary to seek approval for the pivotal trial for enVVe.
Cautionary Note on Forward-Looking Statements
This press release and any statements of stockholders, directors, employees, representatives and partners of enVVeno Medical Corporation (the "Company") related thereto contain, or may contain, among other things, certain "forward-looking statements" within the meaning of the Private Securities Litigation Reform Act of 1995. Such forward-looking statements involve significant risks and uncertainties. Such statements may include, without limitation, statements identified by words such as "projects," "may," "will," "could," "would," "should," "believes," "expects," "anticipates," "estimates," "intends," "plans," "potential" or similar expressions. These statements are based upon the current beliefs and expectations of the Company's management and are subject to significant risks and uncertainties, including those detailed in the Company's filings with the Securities and Exchange Commission. Actual results and timing (may differ significantly from those set forth or implied in the forward-looking statements. Forward-looking statements involve certain risks and uncertainties that are subject to change based on various factors (many of which are beyond the Company's control). The Company undertakes no obligation to publicly update any forward-looking statements, whether as a result of new information, future presentations or otherwise, except as required by applicable law.
INVESTOR CONTACT:
Jenene Thomas, JTC Team, LLC
[email protected]
(908) 824-0775
MEDIA CONTACT:
Glenn Silver, FINN Partners
[email protected]
(973) 818-8198
SOURCE: enVVeno Medical Corporation
View the original press release on ACCESS Newswire
P.Santos--AMWN


