-
 Van der Poel romps to cobbled classic win
Van der Poel romps to cobbled classic win
-
Republicans back Trump, Democrats attack 'illegal' Iran war

-
 Madonna is surprise attraction at Dolce & Gabbana Milan show
Madonna is surprise attraction at Dolce & Gabbana Milan show
-
Farhan keeps Pakistan hopes alive as they post 212-8 against Sri Lanka

-
 Afghanistan says civilians killed in Pakistan air strikes
Afghanistan says civilians killed in Pakistan air strikes
-
Tug of war: how US presidents battle Congress for military powers

-
 Residents flee as Iran missiles stun peaceful Gulf cities
Residents flee as Iran missiles stun peaceful Gulf cities
-
Streets empty and shops close as US strikes confirm Iranian fears

-
 Israelis shelter underground as Iran fires missiles
Israelis shelter underground as Iran fires missiles
-
Bournemouth held by Sunderland in blow to European bid

-
 VAR expanded to include second bookings and corners for World Cup
VAR expanded to include second bookings and corners for World Cup
-
Iranians in Istanbul jittery but jubilant at US, Israeli strikes

-
 Congo-Brazzaville president vows to keep power as campaign kicks off
Congo-Brazzaville president vows to keep power as campaign kicks off
-
US, Israel launch strikes on Iran, Tehran hits back across region

-
 Germany's Aicher wins women's super-G in Soldeu
Germany's Aicher wins women's super-G in Soldeu
-
Fight against terror: Trump threatens Tehran's mullahs

-
 US and Israel launch strikes on Iran, explosions reported across region
US and Israel launch strikes on Iran, explosions reported across region
-
Iran's Khamenei: ruthless revolutionary at apex of Islamic republic

-
 In Iran attack, Trump seeks what he foreswore -- regime change
In Iran attack, Trump seeks what he foreswore -- regime change
-
Climate change forces facelift for Michelangelo masterpiece

-
 Trump says US aims to destroy Iran's military, topple government
Trump says US aims to destroy Iran's military, topple government
-
Acosta wins season-opening MotoGP sprint after Marquez penalty

-
 US and Israel launch strikes against Iran
US and Israel launch strikes against Iran
-
Afghanistan says Pakistan fighter jet down as cross-border strikes flare

-
 Kerr says only '85 percent' fit for Women's Asian Cup
Kerr says only '85 percent' fit for Women's Asian Cup
-
Messi's Inter Miami to visit White House: US media

-
 Thunder beat Nuggets in overtime on Gilgeous-Alexander's return
Thunder beat Nuggets in overtime on Gilgeous-Alexander's return
-
'It's surreal': Zimbabwe superfans revel in unexpected ride to India

-
 New 'Wuthering Heights' film unleashes fresh wave of Bronte-mania
New 'Wuthering Heights' film unleashes fresh wave of Bronte-mania
-
US backs Pakistan's 'right to defend itself' after strikes on Afghanistan

-
 Bezzecchi beats Marquez to pole at season-opening Thailand MotoGP
Bezzecchi beats Marquez to pole at season-opening Thailand MotoGP
-
OpenAI strikes Pentagon deal with 'safeguards' as Trump dumps Anthropic

-
 Oscar-nominated 'F1' sound engineers recreate roar of racetrack
Oscar-nominated 'F1' sound engineers recreate roar of racetrack
-
15 dead as cash-packed military plane crashes in Bolivia

-
 Costa Rica's Grynspan pledges reform in bid for UN chief job
Costa Rica's Grynspan pledges reform in bid for UN chief job
-
Former All Black Bridge hailed for influence at Western Force

-
 'Sinners' vampires inspired by animals, says Oscar hopeful makeup artist
'Sinners' vampires inspired by animals, says Oscar hopeful makeup artist
-
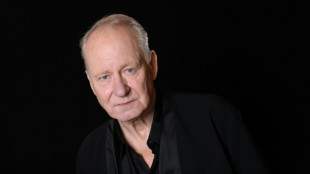
For Oscar nominee Stellan Skarsgard, good cinema is like slow food
-
 'Brilliant industry' sees Reds down Highlanders in Super Rugby
'Brilliant industry' sees Reds down Highlanders in Super Rugby
-
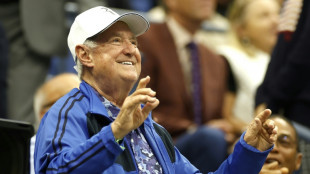
Neil Sedaka, US singer and songwriter, dies age 86
-
 New to The Street to Broadcast Executive Leadership Interviews Featuring Medicus Pharma Ltd. (NASDAQ:MDCX), CitroTech (NYSE:CITR), Vivos Therapeutics, Inc. (NASDAQ:VVOS), and Virtuix Holdings ($VTIX) on Bloomberg Television Tonight at 6:30 PM EST
New to The Street to Broadcast Executive Leadership Interviews Featuring Medicus Pharma Ltd. (NASDAQ:MDCX), CitroTech (NYSE:CITR), Vivos Therapeutics, Inc. (NASDAQ:VVOS), and Virtuix Holdings ($VTIX) on Bloomberg Television Tonight at 6:30 PM EST
-
Understanding Common Dental Issues That Develop Over Time in Richardson, TX

-
 Building Smarter: How Proptech Is Reshaping Canadian Real Estate Development
Building Smarter: How Proptech Is Reshaping Canadian Real Estate Development
-
MEWA Launches the First Saudi Water Week Next April to Shape the Future of the Water Sector Regionally and Globally

-
 Paramount acquires Warner Bros. in $110 bn mega-merger
Paramount acquires Warner Bros. in $110 bn mega-merger
-
Rosenior eyes extended stay to stabilise Chelsea

-
 Spurs struggling physically admits Tudor
Spurs struggling physically admits Tudor
-
Lens held by Strasbourg in blow to Ligue 1 title chances

-
 NFL salary cap passes $300 mn for first time
NFL salary cap passes $300 mn for first time
-
Wolves secure rare win to dent Villa's bid for Champions League place


Aspira Women's Health Provides Update on ARPA-H Sprint Program Partnership to Advance Women's Health
AUSTIN, TX AND WASHINGTON, DC / ACCESS Newswire / June 11, 2025 / Aspira Women's Health Inc., ("Aspira") (OTCQB:AWHL), an AI enhanced bio-analytics based women's health company focused on delivering leading noninvasive gynecologic disease diagnostic and disease management tools, announces an update from the Advanced Research Projects Agency for Health's ( ARPA-H ) Sprint for Women's Health program initiative to address critical unmet needs in women's health.
Aspira was originally awarded a $10 Million award through ARPA-H's Sprint for Women's Health to support the development of Aspira's ENDOinform test, a non-invasive blood draw-based multi-omics test utilizing protein and microRNA biomarkers, along with patient-specific data, to fuel a powerful, AI-powered algorithm to aid in the diagnosis of endometriosis in patients with symptoms of the disease.
This test, leveraging technology and expertise originally developed by Aspira for ovarian cancer risk assessment diagnostics, promises the potential to non-invasively confirm presence of suspected endometriosis in patients, enabling physicians to identify patients who may benefit from endometriosis-controlling therapeutics currently available.
The original ARPA-H contract was announced on October 24, 2024, and reflected $10 million divided into eight payments. The first two of those milestone payments, for $2 million and $1.5 million, were received on November 29, 2024, and March 28, 2025, respectively, for a total of $ 3.5 million from the ARPA-H program. On June 9, 2025, Aspira received notice from ARPA-H that ARPA-H and the assigned managing contractor, VentureWell, have determined that Aspira had not met the specifications of Milestone 3, and have therefore elected to terminate the ENDOinform development program contract.
Aspira's CEO, Mike Buhle, commented, "While we are certainly disappointed in ARPA-H's termination notice, we are highly confident that our team fully achieved all specifications requirements in the Milestone 3 scope of work provisions. We achieved these requirements in early May and are well underway in achieving Milestone 4 targeted specifications. We are confident in our quality of work, and even more so in our understanding of the technical requirements to complete the ENDOinform program."
Buhle further commented, "Our R&D team has been excited about the progress we have made, as well as the early indications of our potential success with the ENDOinform program overall. We plan to continue development of this critical women's health program with the help and support of our shareholders, our research & collaboration partners, and the ongoing dedication of our highly talented and missional teammates. ENDOinform promises a dramatic improvement in health outcomes and quality of life for millions of women suffering from endometriosis, both in the U.S. and abroad. The program remains targeted for 2026 completion goals. We look forward to continuing our development work to completion. In fact, compliance with the ARPA-H program involved significant time investment for our R&D team, and this may well offer us a material acceleration in the pace of achieving our goals."
About Aspira Women's Health Inc.
Aspira Women's Health Inc. is dedicated to the discovery, development, and commercialization of noninvasive, AI-powered tests to aid in the diagnosis of gynecologic diseases. OvaWatch® and Ova1Plus® are offered to clinicians as OvaSuiteSM. Together, they provide the only comprehensive portfolio of blood tests to aid in the detection of ovarian cancer risk for the 1.2+ million American women diagnosed with an adnexal mass each year.
OvaWatch provides a negative predictive value of 99% and is used to assess ovarian cancer risk for women where initial clinical assessment indicates the mass is indeterminate or benign, and thus surgery may be premature or unnecessary. Ova1Plus is a reflex process of two FDA-cleared tests, Ova1® and Overa®, to assess the risk of ovarian malignancy in women with an adnexal mass planned for surgery.
Our in-development test pipeline will expand our ovarian cancer portfolio and address the tremendous need for non-invasive diagnostics for endometriosis, a debilitating disease that impacts millions of women worldwide. In ovarian cancer, we intend to combine microRNA and protein biomarkers with patient data to further enhance the sensitivity and specificity of our current tests. In endometriosis, we have developed the first-ever non-invasive test designed to identify endometriomas, one of the most commonly occurring forms of severe endometriosis. Through our ongoing endometriosis development program, we are combining microRNA and protein biomarkers with patient data, with the intent of identifying all endometriosis independent of disease location or severity.
Forward-Looking Statements
This press release contains forward-looking statements, as defined in the Private Securities Litigation Reform Act of 1995. Forward-looking statements involve a number of risks and uncertainties. Such forward-looking statements include statements regarding, among other things, the timing and completion of any products in the development pipeline and other statements that are predictive in nature, and whether the marketing of the OvaSuite portfolio will prove successful. Actual results could differ materially from those discussed due to known and unknown risks, uncertainties, and other factors. These forward-looking statements generally can be identified by the use of words such as "designed to," "expect," "plan," "anticipate," "could," "may," "intend," "will," "continue," "future," and other words of similar meaning and the use of future dates. These and additional risks and uncertainties are described more fully in the Company's filings with the Securities and Exchange Commission (SEC), including those factors identified as "Risk Factors" in our most recent Annual Report on Form 10-K for the fiscal year ended December 31, 2024, and subsequent Quarterly Reports on Form 10-Q. If any of these risks materialize or our assumptions prove incorrect, actual results could differ materially from the results implied by these forward-looking statements. There may be additional risks that Aspira presently does not know, or that Aspira currently believes are immaterial, that could also cause actual results to differ from those contained in the forward-looking statements. In addition, forward-looking statements reflect Aspira's expectations, plans, or forecasts of future events and views as of the date of this press release. Subsequent events and developments may cause the Company's assessments to change. However, while Aspira may elect to update these forward-looking statements at some point in the future, Aspira expressly disclaims any obligation to do so, except as required by law. These forward-looking statements should not be relied upon as representing Aspira's assessments of any date after the date of this press release. Accordingly, undue reliance should not be placed upon the forward-looking statements.
Investor Relations Contact:
SOURCE: Aspira Women's Health
View the original press release on ACCESS Newswire
P.Santos--AMWN


