-
 Streets empty and shops close as US strikes confirm Iranian fears
Streets empty and shops close as US strikes confirm Iranian fears
-
Israelis shelter underground as Iran fires missiles

-
 Bournemouth held by Sunderland in blow to European bid
Bournemouth held by Sunderland in blow to European bid
-
VAR expanded to include second bookings and corners for World Cup

-
 Iranians in Istanbul jittery but jubilant at US, Israeli strikes
Iranians in Istanbul jittery but jubilant at US, Israeli strikes
-
Congo-Brazzaville president vows to keep power as campaign kicks off

-
 US, Israel launch strikes on Iran, Tehran hits back across region
US, Israel launch strikes on Iran, Tehran hits back across region
-
Germany's Aicher wins women's super-G in Soldeu

-
 Fight against terror: Trump threatens Tehran's mullahs
Fight against terror: Trump threatens Tehran's mullahs
-
US and Israel launch strikes on Iran, explosions reported across region

-
 Iran's Khamenei: ruthless revolutionary at apex of Islamic republic
Iran's Khamenei: ruthless revolutionary at apex of Islamic republic
-
In Iran attack, Trump seeks what he foreswore -- regime change
-
 Climate change forces facelift for Michelangelo masterpiece
Climate change forces facelift for Michelangelo masterpiece
-
Trump says US aims to destroy Iran's military, topple government
-
 Acosta wins season-opening MotoGP sprint after Marquez penalty
Acosta wins season-opening MotoGP sprint after Marquez penalty
-
US and Israel launch strikes against Iran

-
 Afghanistan says Pakistan fighter jet down as cross-border strikes flare
Afghanistan says Pakistan fighter jet down as cross-border strikes flare
-
Kerr says only '85 percent' fit for Women's Asian Cup

-
 Messi's Inter Miami to visit White House: US media
Messi's Inter Miami to visit White House: US media
-
Thunder beat Nuggets in overtime on Gilgeous-Alexander's return

-
 'It's surreal': Zimbabwe superfans revel in unexpected ride to India
'It's surreal': Zimbabwe superfans revel in unexpected ride to India
-
New 'Wuthering Heights' film unleashes fresh wave of Bronte-mania

-
 US backs Pakistan's 'right to defend itself' after strikes on Afghanistan
US backs Pakistan's 'right to defend itself' after strikes on Afghanistan
-
Bezzecchi beats Marquez to pole at season-opening Thailand MotoGP

-
 OpenAI strikes Pentagon deal with 'safeguards' as Trump dumps Anthropic
OpenAI strikes Pentagon deal with 'safeguards' as Trump dumps Anthropic
-
Oscar-nominated 'F1' sound engineers recreate roar of racetrack

-
 15 dead as cash-packed military plane crashes in Bolivia
15 dead as cash-packed military plane crashes in Bolivia
-
Costa Rica's Grynspan pledges reform in bid for UN chief job

-
 Former All Black Bridge hailed for influence at Western Force
Former All Black Bridge hailed for influence at Western Force
-
'Sinners' vampires inspired by animals, says Oscar hopeful makeup artist
-
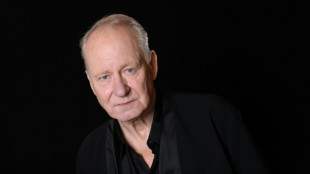 For Oscar nominee Stellan Skarsgard, good cinema is like slow food
For Oscar nominee Stellan Skarsgard, good cinema is like slow food
-
'Brilliant industry' sees Reds down Highlanders in Super Rugby

-
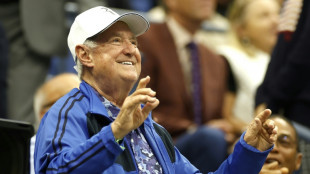 Neil Sedaka, US singer and songwriter, dies age 86
Neil Sedaka, US singer and songwriter, dies age 86
-
Building Smarter: How Proptech Is Reshaping Canadian Real Estate Development

-
 MEWA Launches the First Saudi Water Week Next April to Shape the Future of the Water Sector Regionally and Globally
MEWA Launches the First Saudi Water Week Next April to Shape the Future of the Water Sector Regionally and Globally
-
Paramount acquires Warner Bros. in $110 bn mega-merger

-
 Rosenior eyes extended stay to stabilise Chelsea
Rosenior eyes extended stay to stabilise Chelsea
-
Spurs struggling physically admits Tudor

-
 Lens held by Strasbourg in blow to Ligue 1 title chances
Lens held by Strasbourg in blow to Ligue 1 title chances
-
NFL salary cap passes $300 mn for first time

-
 Wolves secure rare win to dent Villa's bid for Champions League place
Wolves secure rare win to dent Villa's bid for Champions League place
-
Oil prices jump on Iran attack fears while US stocks fall

-
 Two dead, dozens injured as tram derails in Milan
Two dead, dozens injured as tram derails in Milan
-
Trump tells US govt to 'immediately' stop using Anthropic AI tech

-
 Court orders Greenpeace to pay $345 mn to US oil pipeline company
Court orders Greenpeace to pay $345 mn to US oil pipeline company
-
IAEA stresses 'urgency' to verify Iran's nuclear material

-
 UN urges action to prevent full civil war in South Sudan
UN urges action to prevent full civil war in South Sudan
-
Hackers steal medical details of 15 million in France

-
 Susan Sarandon praises Spain’s stance on Gaza
Susan Sarandon praises Spain’s stance on Gaza
-
Murray adamant size isn't everything despite losing Wales place


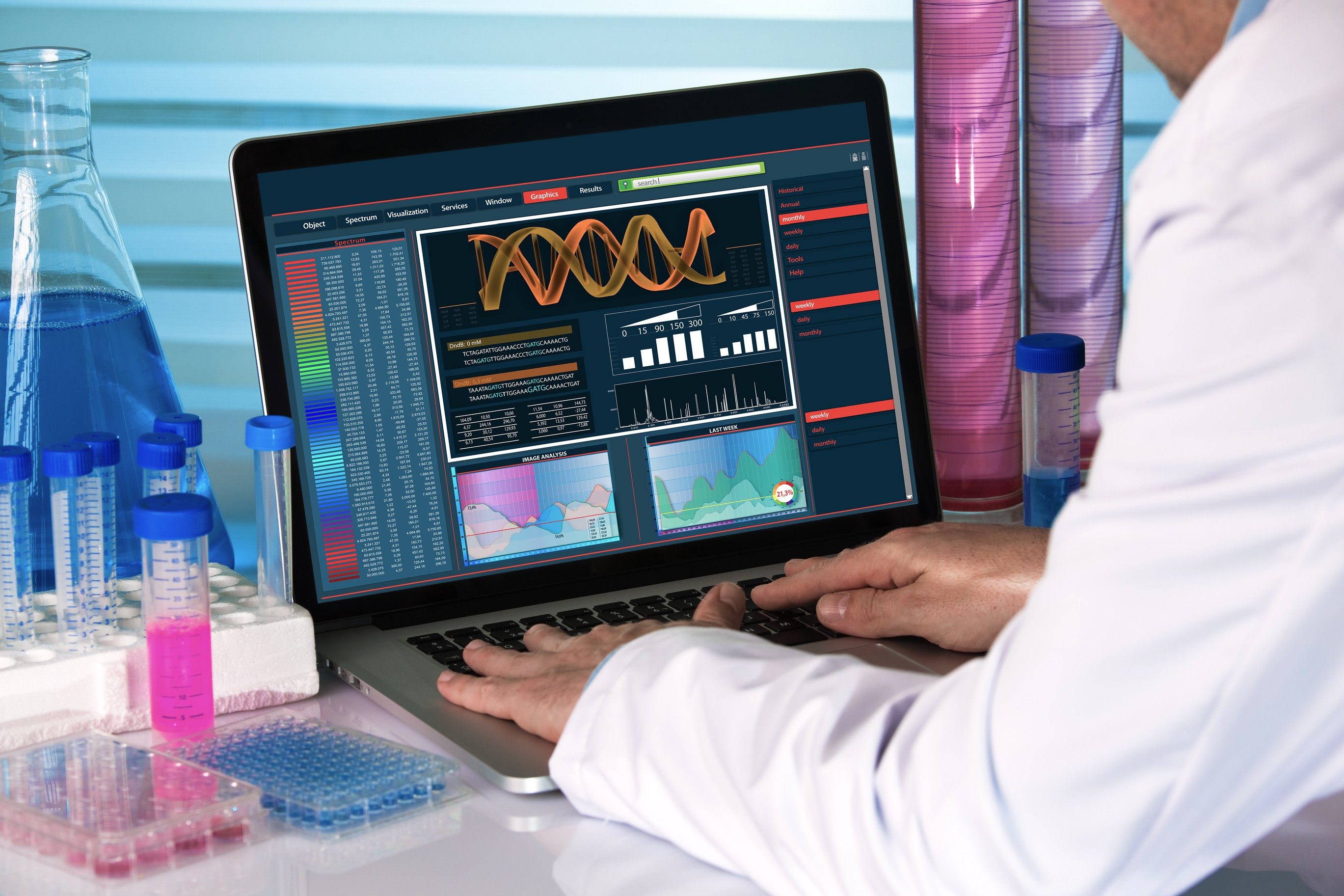
Adolore BioTherapeutics Announces Publication Demonstrating Biosafety and Efficacy of Kv7 Activating rdHSV-CA8* Analgesic Gene Therapy for Chronic Pain via the Intra-Articular Route in Mice
Findings highlight the advantages of Adolore's approach for the delivery of proprietary gene therapy directly to specialized pain-sensing peripheral nerves (nociceptors) that mediates profound analgesia with the potential to address the great unmet need for non-opioid chronic pain therapies
Data further supports the clinical-translational value of Adolore's proprietary non-opioid analgesics for treating chronic non-cancer pain
These data support the Company's continuing efforts to progress with IND-enabling studies of ADB-102 gene therapy for the treatment of osteoarthritis (OA) chronic knee pain.
DELRAY BEACH, FL / ACCESS Newswire / June 15, 2025 / Adolore BioTherapeutics ("Adolore" or the "Company"), a biotechnology company focused on developing breakthrough opioid-free gene therapy treatments for chronic pain and neurological disorders, today announced the publication of its manuscript titled, "Biosafety and Efficacy of Kv7 Activating rdHSV-CA8* Analgesic Gene Therapy for Chronic Pain Via the Intra-Articular Route in Mice1," in the peer-reviewed journal, Molecular Therapy.
Roy Clifford Levitt, MD, Clinical Professor at the University of Miami, Principal Investigator and Program Director of the NIH, NINDS, HEAL Award supporting ADB-102 development for the treatment of chronic knee pain due to OA, and Founder & Executive Chairman of Adolore BioTherapeutics, published biosafety and efficacy data from preclinical studies of Adolore's gene therapy expressing a human carbonic anhydrase-8 variant peptide (CA8*). In model systems, replication-defective, disease-free, herpes simplex virus (rdHSV) gene therapy expressing an analgesic carbonic anhydrase-8 (CA8*) peptide variant corrects somatosensory hyperexcitability by activating Kv7 voltage-gated potassium channels, produces profound, long-lasting analgesia and treats chronic pain from knee OA. In these studies, we provide the first non-Good Laboratory Practices (GLP) biosafety, efficacy, biodistribution, shedding, and histopathology examination of this rdHSV-CA8* via the intra-articular knee route of administration. Naive mice were examined for clinical safety, distribution of virus across all major tissues, knee histopathology, and analgesic efficacy. We observed no signs of persistent toxicity or histopathology, viral genomes remained where they were injected, and there was no evidence of shedding. Profound analgesia persisted for >6 months without functional impairments. These initial biosafety and efficacy data support further development of rdHSV-CA8* for treating chronic knee pain due to moderate-to-severe OA.
Dr. Levitt, commented, "Kv7 voltage-gated potassium channel activators, like rdHSV-CA8* open these channels and hyperpolarize nociceptors making them less excitable to produce profound analgesia. Kv7 activators are well-known to produce potent non-opioid-based analgesia in many human chronic pain conditions. While Kv7 openers are no longer available due to off-target adverse events related to systemic administration, they have been successfully translated from animal models to human chronic pain conditions. Bolstered by our substantial body of published data, we continue to develop our innovative approach to address the significant serious unmet need for safe and effective locally acting pain therapies to replace opioids. Our preclinical data strongly support continued preclinical development toward an IND and clinical studies of ADB-102."
The Company's lead development program for the treatment of chronic pain in knee osteoarthritis is fully funded by a UG3/UH3 grant awarded to the University of Miami by NIH/NINDS HEAL program to support all formal pre-clinical GLP/GMP/GCP development work through a first-in-human study of ADLR-1l01 in patients expected to commence in 2026.
1 Levitt et al., Biosafety and efficacy of Kv7 activating rdHSV-CA8* analgesic gene therapy for chronic pain via the intraarticular route in mice, Molecular Therapy (2025), https://doi.org/10.1016/j.ymthe.2025.05.02
About Carbonic Anhydrase-8 (CA8*) Gene Therapy
CA8* (variants of naturally occurring human carbonic anhydrase-8 analgesic peptides) gene therapies are a novel class of neuronal calcium channel inhibitors that activate Kv7 voltage-gated potassium channels and are administered locally and long-acting. Oral small molecule pain therapeutics that activate Kv7 voltage-gated potassium channels demonstrated proven analgesic efficacy before they were removed from the market due to severe adverse events related to systemic exposure and their metabolism. CA8* gene therapy provides versatile dosing regimens and routes of administration, including intra-articular, intra-neuronal (nerve block), and intradermal injection. This non-opioid CA8* mechanism-of-action addresses neuropathic, inflammatory, and nociceptive pain, which applies to a broad range of chronic pain indications and neurological disorders. These conditions include osteoarthritis, lower back, and cancer pain; diabetes and other forms of peripheral neuropathy; as well as rare pain conditions such as erythromelalgia, a heritable chronic pain condition and epilepsy and hearing loss.
About Adolore BioTherapeutics, Inc.
Adolore BioTherapeutics, Inc., is a biotechnology company focused on developing novel therapies for treating chronic pain using a revolutionary intra-cellular replication-defective HSV (rdHSV) drug delivery platform that is disease-free, non-toxic, and permits localized peripheral nervous system delivery of proprietary biotherapeutics. This rdHSV gene therapy technology incorporates an established re-dosing strategy and an excellent safety profile. HSV vectors are known for their stability and prolonged gene expression, providing an excellent basis for the long- term treatment of chronic pain conditions and neurological disorders. Our best-in-class CA8* programs are long-acting, locally administered gene therapies that are opioid-free Disease-Modifying Anti-Pain therapies (DMAPs) designed to treat many forms of chronic pain, epilepsy and hearing loss.
Leveraging its innovative gene therapy vectors expressing CA8* analgesic peptides (ADLR-1001), Adolore is currently advancing two preclinical development programs: ADB-101 for the treatment of patients' chronic pain caused by erythromelalgia, an orphan disease, and ADB-102, their lead program for the treatment of patients with chronic pain caused by knee OA. Based on substantial compelling preclinical data generated to date, the Company is progressing these programs toward IND filings and first-in-human clinical studies. Adolore has two additional programs: ADB-104 for Drug-Resistant Refractory Focal Epilepsy and ADB-105 for Acute Severe Hearing Loss.
For more information, visit adolore.com.
Forward-Looking Statements
To the extent, this announcement contains information and statements that are not historical, they are considered forward-looking statements within the meaning of the federal securities laws. You can identify forward-looking statements by the use of the words "believe," "expect," "anticipate," "intend," "estimate," "project," "will," "should," "may," "plan," "intend," "assume" and other expressions which predict or indicate future events and trends and which do not relate to historical matters. You should not rely on forward-looking statements because they involve known and unknown risks, uncertainties, and other factors, some of which are beyond the control of the Company. These risks and uncertainties include but are not limited to those associated with drug development. These risks, uncertainties, and other factors may cause the actual results, performance, or achievements of the Company to be materially different from the anticipated future results, performance, or achievements expressed or implied by the forward- looking statements.
Investor Relations Contact
Paul Barone (215)622-4542
[email protected]
SOURCE: Adolore Biotherapeutics, Inc.
View the original press release on ACCESS Newswire
S.Gregor--AMWN


