-
 US, Israel launch strikes on Iran, Tehran hits back across region
US, Israel launch strikes on Iran, Tehran hits back across region
-
Germany's Aicher wins women's super-G in Soldeu

-
 Fight against terror: Trump threatens Tehran's mullahs
Fight against terror: Trump threatens Tehran's mullahs
-
US and Israel launch strikes on Iran, explosions reported across region

-
 Iran's Khamenei: ruthless revolutionary at apex of Islamic republic
Iran's Khamenei: ruthless revolutionary at apex of Islamic republic
-
In Iran attack, Trump seeks what he foreswore -- regime change
-
 Climate change forces facelift for Michelangelo masterpiece
Climate change forces facelift for Michelangelo masterpiece
-
Trump says US aims to destroy Iran's military, topple government
-
 Acosta wins season-opening MotoGP sprint after Marquez penalty
Acosta wins season-opening MotoGP sprint after Marquez penalty
-
US and Israel launch strikes against Iran

-
 Afghanistan says Pakistan fighter jet down as cross-border strikes flare
Afghanistan says Pakistan fighter jet down as cross-border strikes flare
-
Kerr says only '85 percent' fit for Women's Asian Cup

-
 Messi's Inter Miami to visit White House: US media
Messi's Inter Miami to visit White House: US media
-
Thunder beat Nuggets in overtime on Gilgeous-Alexander's return

-
 'It's surreal': Zimbabwe superfans revel in unexpected ride to India
'It's surreal': Zimbabwe superfans revel in unexpected ride to India
-
New 'Wuthering Heights' film unleashes fresh wave of Bronte-mania

-
 US backs Pakistan's 'right to defend itself' after strikes on Afghanistan
US backs Pakistan's 'right to defend itself' after strikes on Afghanistan
-
Bezzecchi beats Marquez to pole at season-opening Thailand MotoGP

-
 OpenAI strikes Pentagon deal with 'safeguards' as Trump dumps Anthropic
OpenAI strikes Pentagon deal with 'safeguards' as Trump dumps Anthropic
-
Oscar-nominated 'F1' sound engineers recreate roar of racetrack

-
 15 dead as cash-packed military plane crashes in Bolivia
15 dead as cash-packed military plane crashes in Bolivia
-
Costa Rica's Grynspan pledges reform in bid for UN chief job

-
 Former All Black Bridge hailed for influence at Western Force
Former All Black Bridge hailed for influence at Western Force
-
'Sinners' vampires inspired by animals, says Oscar hopeful makeup artist
-
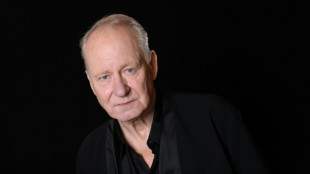 For Oscar nominee Stellan Skarsgard, good cinema is like slow food
For Oscar nominee Stellan Skarsgard, good cinema is like slow food
-
'Brilliant industry' sees Reds down Highlanders in Super Rugby

-
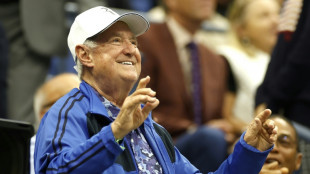 Neil Sedaka, US singer and songwriter, dies age 86
Neil Sedaka, US singer and songwriter, dies age 86
-
Building Smarter: How Proptech Is Reshaping Canadian Real Estate Development

-
 MEWA Launches the First Saudi Water Week Next April to Shape the Future of the Water Sector Regionally and Globally
MEWA Launches the First Saudi Water Week Next April to Shape the Future of the Water Sector Regionally and Globally
-
Paramount acquires Warner Bros. in $110 bn mega-merger

-
 Rosenior eyes extended stay to stabilise Chelsea
Rosenior eyes extended stay to stabilise Chelsea
-
Spurs struggling physically admits Tudor

-
 Lens held by Strasbourg in blow to Ligue 1 title chances
Lens held by Strasbourg in blow to Ligue 1 title chances
-
NFL salary cap passes $300 mn for first time

-
 Wolves secure rare win to dent Villa's bid for Champions League place
Wolves secure rare win to dent Villa's bid for Champions League place
-
Oil prices jump on Iran attack fears while US stocks fall

-
 Two dead, dozens injured as tram derails in Milan
Two dead, dozens injured as tram derails in Milan
-
Trump tells US govt to 'immediately' stop using Anthropic AI tech

-
 Court orders Greenpeace to pay $345 mn to US oil pipeline company
Court orders Greenpeace to pay $345 mn to US oil pipeline company
-
IAEA stresses 'urgency' to verify Iran's nuclear material

-
 UN urges action to prevent full civil war in South Sudan
UN urges action to prevent full civil war in South Sudan
-
Hackers steal medical details of 15 million in France

-
 Susan Sarandon praises Spain’s stance on Gaza
Susan Sarandon praises Spain’s stance on Gaza
-
Murray adamant size isn't everything despite losing Wales place

-
 Messi knocked down by fan in Puerto Rico pitch invasion
Messi knocked down by fan in Puerto Rico pitch invasion
-
Two killed, dozens injured as tram derails in Milan

-
 O'Neill taken aback by Rangers boss Rohl's comments on Celtic
O'Neill taken aback by Rangers boss Rohl's comments on Celtic
-
Ukrainian, Slovak leaders hold call amid energy spat

-
 French hard-left firebrand sparks row with 'antisemitic' Epstein jibe
French hard-left firebrand sparks row with 'antisemitic' Epstein jibe
-
Ahmed, Jacks blast England to thrilling win over New Zealand


Aspira Women's Health Partners with Dorsata to Offer Access to 300+ Women's Health Practices, 1.5 Million + New Patients
Aspira and Dorsata Launch Clinical Workflow Tool for Adnexal Masses,
Now Live for All Dorsata Clients, Adnexal Mass Evidence-Based Decision Support
AUSTIN, TX AND WASHINGTON, DC / ACCESS Newswire / June 17, 2025 / Aspira Women's Health Inc. a bio-analytical-based women's health company focused on gynecologic disease diagnostics, and Dorsata, a leading clinical decision-support and provider workflow platform for women's health, announced today the official launch of a new adnexal mass clinical decision support module in partnership with Dorsata, a leading women's health electronic health record software solutions company.
Dorsata's platform is currently used by more than 700 women's health providers across over 300 practice sites in 20 states, helping clinicians standardize care delivery and improve outcomes through guided clinical workflows and real-time decision support for over 1.5 million patients each year. The module is now live and available as of June 2, 2025, to all Dorsata clients nationwide.
"I am very pleased to announce such an important accomplishment for our team," commented Mike Buhle, CEO of Aspira. "We are extremely focused on growing our Ova1Plus® and OvaWatch® adoption in the most efficient, accelerated way possible. Our partnership with Dorsata is an excellent example of our revised go-to-market strategy which brings real value to providers, patients, and Aspira alike."
"Today, we are currently completing approximately 24,000 tests annually and 4,000 providers actively prescribing Ova1Plus® and OvaWatch® at any one time. Our partnership with Dorsata increases our access to providers by 700. That represents a 17.5% increase in providers."
"We estimate there are approximately 1.2 million instances where care providers uncover an ovarian mass every year. With approximately 30,000 OBGYNs in the US today, that results in about 40 times per year where the average care provider has the opportunity to prescribe the Ova1Plus® and OvaWatch® test. The Dorsata module offers evidence-based, clinical decision support at the point-of-care to make sure that no patient is overlooked. This results in approximately 28,000 instances where our test may positively impact a women's health. This is a tremendous opportunity for our team to make a difference. Across a much broader addressable market."
Dorsata co-founder & CEO, David Fairbrothers, said in a statement "from the earliest days of Dorsata, our mission has been to present relevant, evidence-based guidelines at the point of care in actionable ways that will improve patient care. Our partnership with Aspira is a great example of how this vision brings value to providers and to women alike."
"This is an excellent example of how we want to quickly leverage technology and strategic relationships to reach more patients and positively impact women's heath, while creating lasting shareholder value. I am extremely proud of our team for contributing to such a critical milestone for Aspira," concluded Mr. Buhle.
Additional Information Regarding Aspira - Dorsata Launch
This innovative module is embedded directly within Dorsata's clinical workflow platform and empowers OB/GYN providers to assess and manage patients presenting with adnexal masses using a consistent, structured approach. It integrates Aspira's blood tests - Ova1Plus® and OvaWatch® - to support timely and accurate risk stratification for ovarian malignancy.
The adnexal mass module was designed collaboratively by clinical, product, and workflow experts from both organizations to align with best practices for managing adnexal masses. Providers using the tool can seamlessly order and document Aspira's OvaSuite tests without disrupting their daily workflow, enhancing clinical efficiency and patient safety.
The new module reinforces both companies' missions to modernize women's health care through smarter, tech-enabled clinical tools that support early detection, precision diagnostics, and better outcomes. The adnexal mass module is now available. For more information or to schedule a demo, please contact Dorsata or Aspira Women's Health directly.
About Aspira Women's Health Inc.
Aspira Women's Health Inc. is dedicated to the discovery, development, and commercialization of noninvasive, AI-powered tests to aid in the diagnosis of gynecologic diseases. OvaWatch® and Ova1Plus® are offered to clinicians as OvaSuiteSM. Together, they provide the only comprehensive portfolio of blood tests to aid in the detection of ovarian cancer risk for the 1.2+ million American women diagnosed with an adnexal mass each year.
OvaWatch provides a negative predictive value of 99% and is used to assess ovarian cancer risk for women where initial clinical assessment indicates the mass is indeterminate or benign, and thus surgery may be premature or unnecessary. Ova1Plus is a reflex process of two FDA-cleared tests, Ova1® and Overa®, to assess the risk of ovarian malignancy in women with an adnexal mass planned for surgery.
Our in-development test pipeline will expand our ovarian cancer portfolio and address the tremendous need for non-invasive diagnostics for endometriosis, a debilitating disease that impacts millions of women worldwide. In ovarian cancer, we intend to combine microRNA and protein biomarkers with patient data to further enhance the sensitivity and specificity of our current tests. In endometriosis, we have developed the first-ever non-invasive test designed to identify endometriomas, one of the most commonly occurring forms of severe endometriosis. Through our ongoing endometriosis development program, we are combining microRNA and protein biomarkers with patient data, with the intent of identifying all endometriosis independent of disease location or severity.
Forward-Looking Statements
This press release contains forward-looking statements, as defined in the Private Securities Litigation Reform Act of 1995. Forward-looking statements involve a number of risks and uncertainties. Such forward-looking statements include statements regarding, among other things, the timing and completion of any products in the development pipeline and other statements that are predictive in nature, and whether the marketing of the OvaSuite portfolio will prove successful. Actual results could differ materially from those discussed due to known and unknown risks, uncertainties, and other factors. These forward-looking statements generally can be identified by the use of words such as "designed to," "expect," "plan," "anticipate," "could," "may," "intend," "will," "continue," "future," and other words of similar meaning and the use of future dates. These and additional risks and uncertainties are described more fully in the Company's filings with the Securities and Exchange Commission (SEC), including those factors identified as "Risk Factors" in our most recent Annual Report on Form 10-K for the fiscal year ended December 31, 2024, and subsequent Quarterly Reports on Form 10-Q. If any of these risks materialize or our assumptions prove incorrect, actual results could differ materially from the results implied by these forward-looking statements. There may be additional risks that Aspira presently does not know, or that Aspira currently believes are immaterial, that could also cause actual results to differ from those contained in the forward-looking statements. In addition, forward-looking statements reflect Aspira's expectations, plans, or forecasts of future events and views as of the date of this press release. Subsequent events and developments may cause the Company's assessments to change. However, while Aspira may elect to update these forward-looking statements at some point in the future, Aspira expressly disclaims any obligation to do so, except as required by law. These forward-looking statements should not be relied upon as representing Aspira's assessments of any date after the date of this press release. Accordingly, undue reliance should not be placed upon the forward-looking statements.
Investor Relations Contact:
SOURCE;: Aspira Women's Health
View the original press release on ACCESS Newswire
Ch.Kahalev--AMWN


