
-
 US, Israel launch strikes on Iran, Tehran hits back across region
US, Israel launch strikes on Iran, Tehran hits back across region
-
Germany's Aicher wins women's super-G in Soldeu

-
 Fight against terror: Trump threatens Tehran's mullahs
Fight against terror: Trump threatens Tehran's mullahs
-
US and Israel launch strikes on Iran, explosions reported across region

-
 Iran's Khamenei: ruthless revolutionary at apex of Islamic republic
Iran's Khamenei: ruthless revolutionary at apex of Islamic republic
-
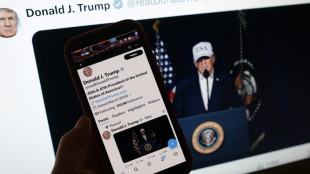
In Iran attack, Trump seeks what he foreswore -- regime change
-
 Climate change forces facelift for Michelangelo masterpiece
Climate change forces facelift for Michelangelo masterpiece
-
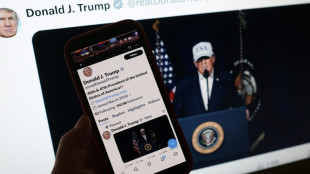
Trump says US aims to destroy Iran's military, topple government
-
 Acosta wins season-opening MotoGP sprint after Marquez penalty
Acosta wins season-opening MotoGP sprint after Marquez penalty
-
US and Israel launch strikes against Iran

-
 Afghanistan says Pakistan fighter jet down as cross-border strikes flare
Afghanistan says Pakistan fighter jet down as cross-border strikes flare
-
Kerr says only '85 percent' fit for Women's Asian Cup

-
 Messi's Inter Miami to visit White House: US media
Messi's Inter Miami to visit White House: US media
-
Thunder beat Nuggets in overtime on Gilgeous-Alexander's return

-
 'It's surreal': Zimbabwe superfans revel in unexpected ride to India
'It's surreal': Zimbabwe superfans revel in unexpected ride to India
-
New 'Wuthering Heights' film unleashes fresh wave of Bronte-mania

-
 US backs Pakistan's 'right to defend itself' after strikes on Afghanistan
US backs Pakistan's 'right to defend itself' after strikes on Afghanistan
-
Bezzecchi beats Marquez to pole at season-opening Thailand MotoGP

-
 OpenAI strikes Pentagon deal with 'safeguards' as Trump dumps Anthropic
OpenAI strikes Pentagon deal with 'safeguards' as Trump dumps Anthropic
-
Oscar-nominated 'F1' sound engineers recreate roar of racetrack

-
 15 dead as cash-packed military plane crashes in Bolivia
15 dead as cash-packed military plane crashes in Bolivia
-
Costa Rica's Grynspan pledges reform in bid for UN chief job

-
 Former All Black Bridge hailed for influence at Western Force
Former All Black Bridge hailed for influence at Western Force
-
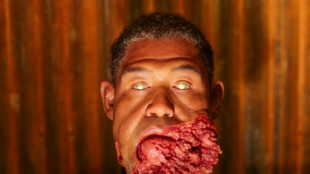
'Sinners' vampires inspired by animals, says Oscar hopeful makeup artist
-
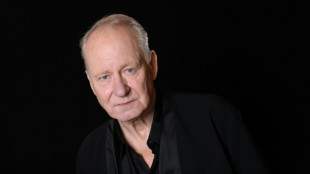 For Oscar nominee Stellan Skarsgard, good cinema is like slow food
For Oscar nominee Stellan Skarsgard, good cinema is like slow food
-
'Brilliant industry' sees Reds down Highlanders in Super Rugby

-
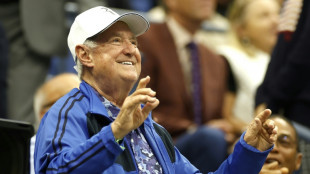 Neil Sedaka, US singer and songwriter, dies age 86
Neil Sedaka, US singer and songwriter, dies age 86
-
Building Smarter: How Proptech Is Reshaping Canadian Real Estate Development

-
 MEWA Launches the First Saudi Water Week Next April to Shape the Future of the Water Sector Regionally and Globally
MEWA Launches the First Saudi Water Week Next April to Shape the Future of the Water Sector Regionally and Globally
-
Paramount acquires Warner Bros. in $110 bn mega-merger

-
 Rosenior eyes extended stay to stabilise Chelsea
Rosenior eyes extended stay to stabilise Chelsea
-
Spurs struggling physically admits Tudor

-
 Lens held by Strasbourg in blow to Ligue 1 title chances
Lens held by Strasbourg in blow to Ligue 1 title chances
-
NFL salary cap passes $300 mn for first time

-
 Wolves secure rare win to dent Villa's bid for Champions League place
Wolves secure rare win to dent Villa's bid for Champions League place
-
Oil prices jump on Iran attack fears while US stocks fall

-
 Two dead, dozens injured as tram derails in Milan
Two dead, dozens injured as tram derails in Milan
-
Trump tells US govt to 'immediately' stop using Anthropic AI tech

-
 Court orders Greenpeace to pay $345 mn to US oil pipeline company
Court orders Greenpeace to pay $345 mn to US oil pipeline company
-
IAEA stresses 'urgency' to verify Iran's nuclear material

-
 UN urges action to prevent full civil war in South Sudan
UN urges action to prevent full civil war in South Sudan
-
Hackers steal medical details of 15 million in France

-
 Susan Sarandon praises Spain’s stance on Gaza
Susan Sarandon praises Spain’s stance on Gaza
-
Murray adamant size isn't everything despite losing Wales place

-
 Messi knocked down by fan in Puerto Rico pitch invasion
Messi knocked down by fan in Puerto Rico pitch invasion
-
Two killed, dozens injured as tram derails in Milan

-
 O'Neill taken aback by Rangers boss Rohl's comments on Celtic
O'Neill taken aback by Rangers boss Rohl's comments on Celtic
-
Ukrainian, Slovak leaders hold call amid energy spat

-
 French hard-left firebrand sparks row with 'antisemitic' Epstein jibe
French hard-left firebrand sparks row with 'antisemitic' Epstein jibe
-
Ahmed, Jacks blast England to thrilling win over New Zealand


Lobe Sciences Moves Forward with Development of Conjugated Psilocin(TM) Following April 14 Agreement to Spin Out Compound to Cynaptec Pharmaceuticals
New subsidiary, Cynaptec Pharmaceuticals, established to hold global rights to Conjugated Psilocin™
$6 million investment in Cynaptec Pharmaceuticals secured to advance research and early-stage clinical trials for chronic cluster headaches and addiction
Option for an additional $20 million investment in Cynaptec Pharmaceuticals to support Phase 3 clinical development
VANCOUVER, BRITISH COLUMBIA / ACCESS Newswire / June 17, 2025 / Lobe Sciences Ltd., (CSE:LOBE)(OTCQB:LOBEF)(FWB:LOBE.F) a leading biopharmaceutical company focused on breakthrough treatments for rare diseases, is advancing the development of its novel compound, Conjugated Psilocin™, following the April 14, 2025 spinout of the compound to the newly created Delaware corporation Cynaptec Pharmaceuticals, Inc. and signing of a key US$6 million investment non-brokered private placement agreement for preferred shares in Cynaptec Pharmaceuticals.
This funding supports preclinical research, Phase 1, and Phase 2a clinical trials as Lobe Sciences Ltd., via Cynaptec Pharmaceuticals, progresses toward new treatment options for chronic cluster headaches, one of the most painful neurological conditions and considered an Orphan Disease. In addition, an option for a further US$20 million investment remains available, which would support the Phase 3 clinical program. Chronic cluster headache affects over 60,000 people in the United States. Currently, there is no FDA-approved drug specifically for chronic cluster headaches. The only FDA-approved medication for clusterheadaches is Emgality (galcanezumab-glnm), but it is indicated only for episodic cluster headaches, not the chronic form. For chronic cluster headaches, treatment typically involves off-label use of medications such as verapamil at very high and potentially dangerous doses, lithium, corticosteroids, and oxygen therapy.
As part of this strategy, Lobe launched Cynaptec Pharmaceuticals, Inc., a Delaware-based private subsidiary now holding the worldwide rights and intellectual property for Conjugated Psilocin™. Lobe transferred all relevant IP and commercial agreements to Cynaptec while retaining a 64% ownership stake after the initial $6 million investment.
Conjugated Psilocin™ is a stable, oral analog of psilocin (the active component of psilocybin) designed to deliver enhanced bioavailability and effectiveness without hallucinations. While the primary focus is treating chronic cluster headaches, ongoing research is also exploring its potential for substance use disorders, expanding its impact.
Lobe Sciences Ltd. is also advancing the introduction of Altemia® Medical Food, a specially formulated oral emulsion designed to address long-chain fatty acid deficiencies commonly seen in Sickle Cell Disease (SCD). SCD affects approximately 100,000 people in the United States and an estimated 10 million worldwide. African American children are disproportionately impacted, with 1 in every 365 African American births affected by the disease. Middle Eastern and South Asian populations also face a heightened risk. Altemia Medical Food has undergone clinical testing, demonstrating its ability to positively impact fatty acid imbalances in patients with SCD, potentially improving overall health. Currently, few treatment options exist for hematologists managing this condition, making Altemia® a promising new approach. This first-of-its-kind medical food is available only under physician supervision, ensuring proper dietary management for those in need. Lobe Sciences Ltd. remains committed to expanding access to innovative solutions for underserved patient populations.
About Lobe Sciences, Ltd. (CSE:LOBE)(OTCQB:LOBEF)(FWB:LOBE.F) - Lobe Sciences is a biopharmaceutical company focused on using lipid technology to develop innovative treatments for orphan and rare diseases. Lobe Sciences commercializes Altemia® MF, a medical food designed to manage deficiencies commonly found in sickle cell disease. Additionally, through Cynaptec, Lobe Sciences retains an interest in developing Conjugated PsilocinTM in neurology and psychiatric indications. Alera Pharma, Inc. remains a wholly owned subsidiary of the Company that Lobe Sciences may elect to utilize for future activity unrelated to Conjugated PsilocinTM.
About Cynaptec Pharmaceuticals, Inc. - Cynaptec is a biopharmaceutical company dedicated to developing innovative therapies for neurological and psychiatric disorders. Cynaptec's initial development program is focused on the use of its proprietary Conjugated PsilocinTM compound for treatment of the significant unmet medical needs of patients with Chronic Cluster Headache, with an additional preliminary proof-of-concept to assess potential utility for substance use disorders.
About Conjugated PsilocinTM- Conjugated PsilocinTM is a novel, patented, oral, stable analog of psilocin, the active metabolite of the prodrug psilocybin, designed to enhance bioavailability and therapeutic efficacy, which has been identified as having therapeutic potential in a variety of neurological conditions. Whereas conventional psilocin is an unstable compound that has been challenging for the industry to develop as a standalone pharmaceutical, Conjugated Psilocin's™ stability and bioavailability profile, and associated safety and efficacy signals, suggest the potential for prescription drug development in a variety of neurological and psychiatric indications.
About Altemia® Medical Food - Altemia Medical Food is a specially formulated emulsified blend of long-chain polyunsaturated fatty acids designed for oral administration in patients with Sickle Cell Disease. As a medical food, Altemia must be prescribed under the care of a physician to ensure appropriate dietary management. Its formulation includes natural emulsifying agents such as egg yolk and Vitamin E, which aid in restoring red blood cell membrane fatty acids. Clinical studies have demonstrated that Altemia Medical Food can help address the fatty acid imbalance frequently observed in Sickle Cell Disease, potentially improving patient health outcomes. With limited treatment options available to hematologists for managing this condition, Altemia provides a promising new approach to supporting individuals affected by this debilitating disease.
For further information:
Dr. Fred D. Sancilio, CEO
Lobe Sciences Ltd.
Investor and Media
[email protected]
Phone: +1 (949) 505-5623
Cautionary Statement Regarding "Forward-Looking" Information
This news release includes certain statements and information that may constitute forward-looking information within the meaning of applicable Canadian securities laws. All statements in this news release, other than statements of historical facts, including statements regarding future estimates, plans, objectives, timing, assumptions or expectations of future performance, including, without limitation: the Company's intention to raise funds for clinical trials, the innovativeness of Conjugated PsilocinTM. the anticipated focus and timing or efficiency of the Company's research and development activities; the potential for the Company's products to meet unmet medical needs, the anticipated use of proceeds from the offering, the intended development plans for Cynaptec, the intention of Lobe Sciences to provide certain services to Cynaptec, the potential exercise of the option and intended use of proceeds therefrom and the potential ability to raise funds for clinical development are forward-looking statements and contain forward-looking information. Generally, forward-looking statements and information can be identified by the use of forward-looking terminology such as "intends" or "anticipates", or variations of such words and phrases or statements that certain actions, events or results "may", "could", "should" or "would" or occur.
Forward-looking statements are based on certain material assumptions and analysis made by the Company and the opinions and estimates of management as of the date of this press release, including, among other things, that: the Company's planned activities will be able to create shareholder value and address serious unmet medical needs; the Company will continue to pursue its planned research and development activities, and that the Company will be able to raise funds among others. These forward-looking statements are subject to known and unknown risks, uncertainties and other factors that may cause the actual results, level of activity, performance or achievements of the Company to be materially different from those expressed or implied by such forward-looking statements or forward-looking information. Important risks that may cause actual results to vary, include, without limitation, the risk that: the Company's planned activities will be unable to create shareholder value or address the targeted unmet medical needs; the Company is unable to obtain the desired results from its current research and development activities; and the Company is unable to raise funds or to do so on the timelines anticipated.
Although management of the Company has attempted to identify important factors that could cause actual results to differ materially from those contained in forward-looking statements or forward-looking information, there may be other factors that cause results not to be as anticipated, estimated or intended. There can be no assurance that such statements will prove to be accurate, as actual results and future events could differ materially from those anticipated in such statements. Accordingly, readers should not place undue reliance on forward-looking statements and forward-looking information. Readers are cautioned that reliance on such information may not be appropriate for other purposes. The Company does not undertake to update any forward-looking statement, forward-looking information or financial out-look that are incorporated by reference herein, except in accordance with applicable securities laws.
NEITHER THE CANADIAN SECURITIES EXCHANGE NOR ITS REGULATION SERVICES PROVIDER HAVE REVIEWED OR ACCEPT RESPONSIBILITY FOR THE ACCURACY OR ADEQUACY OF THIS NEWS RELEASE.
SOURCE: Lobe Sciences Ltd.
View the original press release on ACCESS Newswire
B.Finley--AMWN


