-
 Rubio meets Caribbean leaders as US raises pressure on Cuba
Rubio meets Caribbean leaders as US raises pressure on Cuba
-
Head of France's Versailles Palace to take over Louvre: source to AFP

-
 England's Brook gains redemption after 'hardest winter of my life'
England's Brook gains redemption after 'hardest winter of my life'
-
Iran dismisses missile, nuclear claims after Trump alleges 'sinister ambitions'

-
 Inside the Mexican resort that was the final hideout of 'El Mencho'
Inside the Mexican resort that was the final hideout of 'El Mencho'
-
Somaliland pins hopes on critical mineral gold rush

-
 Bejart Ballet's iconic Bolero ignites Istanbul
Bejart Ballet's iconic Bolero ignites Istanbul
-
Sri Lanka arrests ex-spy chief over 2019 Easter bombings

-
 South Korea birth rate jumps but still under key fertility threshold
South Korea birth rate jumps but still under key fertility threshold
-
Democrats bet on centrism in rebuttal to Trump speech

-
 Australian police arrest two over alleged kidnapping, murder of grandfather
Australian police arrest two over alleged kidnapping, murder of grandfather
-
Redknapp's Gold Cup dream sparked by late grandmother

-
 Trump tries to reset presidency in State of the Union speech
Trump tries to reset presidency in State of the Union speech
-
Harden hails 'special' Cavs after emphatic win over Knicks

-
 Division, theater and one golden moment as Trump addresses Congress
Division, theater and one golden moment as Trump addresses Congress
-
Humble Japan ready to win hearts at Women's Asian Cup

-
 New Zealand mayor swims to allay sewage contamination fears
New Zealand mayor swims to allay sewage contamination fears
-
Trump vows 'turnaround for the ages' in State of the Union

-
 Marquez targets eighth MotoGP title as season opens in Thailand
Marquez targets eighth MotoGP title as season opens in Thailand
-
Months after floods, Indonesian survivors frustrated by slow response

-
 Tech firms lead Asian markets rally as Seoul, Tokyo hit records
Tech firms lead Asian markets rally as Seoul, Tokyo hit records
-
Nepali migrant workers influence polls, but can't vote

-
 Canadians are choosing when to die, often with a smile
Canadians are choosing when to die, often with a smile
-
Trump to promise 'turnaround for the ages' in State of the Union

-
 Economy not Russia is big fear on Finland's closed frontier
Economy not Russia is big fear on Finland's closed frontier
-
Alexandria bids farewell to historic tram in latest urban upheaval

-
 The veteran 'insider' shaping Iran's nuclear policy
The veteran 'insider' shaping Iran's nuclear policy
-
'Jaws' harpoon gun and 'Star Wars' treasures lead LA film and TV auction

-
 EQS Group Partners with Ground Truth Intelligence to Strengthen Enhanced Due Diligence Capabilities in the Compliance Cockpit
EQS Group Partners with Ground Truth Intelligence to Strengthen Enhanced Due Diligence Capabilities in the Compliance Cockpit
-
Chancery Royalty Secures US$20 Million Royalty with KEFI Gold & Copper Plc for Tulu Kapi Gold Project

-
 Noram Adds Additional Critical Mineral to List of High-Value Byproduct Credits in Zeus Project Upgraded PEA
Noram Adds Additional Critical Mineral to List of High-Value Byproduct Credits in Zeus Project Upgraded PEA
-
InterContinental Hotels Group PLC Announces Transaction in Own Shares - February 25

-
 Pentixapharm Receives FDA "Study May Proceed" Letters for Dual Theranostic INDs in CXCR4-Based Hemato-Oncology Program
Pentixapharm Receives FDA "Study May Proceed" Letters for Dual Theranostic INDs in CXCR4-Based Hemato-Oncology Program
-
Tocvan Builds Momentum at Gran Pilar: Six Exploration Holes Drilled, Drone Mag Survey and Water Monitoring Wells for Pilot Completed

-
 Brazil prosecutor urges politicians' conviction in murder of black councilwoman
Brazil prosecutor urges politicians' conviction in murder of black councilwoman
-
Starved of fuel, Cubans scramble to make ends meet

-
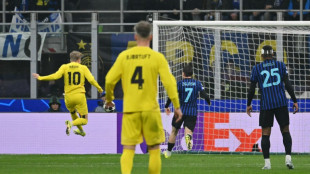
 Giant killers Bodo/Glimt continue remarkable rise with Inter triumph
Giant killers Bodo/Glimt continue remarkable rise with Inter triumph
-
Bodo/Glimt knock Inter out of Champions League as Newcastle, Atletico reach last 16

-
 Australian Open chief Tiley steps down to take top US job
Australian Open chief Tiley steps down to take top US job
-
Crime capital no more: El Salvador tourism boosted by Bukele

-
 FIFA boss 'very reassured' about World Cup in Mexico despite violence
FIFA boss 'very reassured' about World Cup in Mexico despite violence
-
15 states sue Trump administration over child vaccine policy

-
 Rescuers search for missing after deluge kills 30 in Brazil
Rescuers search for missing after deluge kills 30 in Brazil
-
Newcastle complete cruise into Champions League last 16

-
 Leverkusen through to Champions League last 16 after Olympiacos draw
Leverkusen through to Champions League last 16 after Olympiacos draw
-
Bodo/Glimt sink Inter to continue Champions League fairy tale
-
 Tech shares rebound as markets weigh AI impacts
Tech shares rebound as markets weigh AI impacts
-
Puerto Vallarta: the Mexican paradise in flames over the killing of 'El Mencho'

-
 Sorloth treble helps Atletico past Brugge into Champions League last 16
Sorloth treble helps Atletico past Brugge into Champions League last 16
-
Louvre president hands in resignation to Macron: Elysee


enVVeno Medical Updates Regulatory Status of VenoValve(R)
IRVINE, CA / ACCESS Newswire / September 15, 2025 / enVVeno Medical Corporation (Nasdaq:NVNO) ("enVVeno" or the "Company"), a company setting new standards of care for the treatment of deep venous disease, today announced that it will file a request for supervisory appeal of the not-approvable letter from the Center for Devices and Radiological Health (CDRH) of the U.S. Food & Drug Administration (FDA) received on August 19, 2025, in response to its Premarket Approval (PMA) application for the VenoValve®, a surgical replacement venous valve for treating severe deep chronic venous insufficiency (CVI).

The FDA provides several internal informal and formal mechanisms to challenge lower review staff decisions, including scientific controversies. One mechanism is a request for supervisory review in which an appeal is made to the next line of supervision. Supervisory appeals are required to be filed within 30 days of the decision being appealed, which is on or before September 18, 2025. These appeals involve a formal substantive request, an in-person meeting, and a decision. It also often includes multiple interactions even after an initial appeal decision is made.
"Due to our interaction with FDA to obtain our Breakthrough Device Designation (BDD), in which the FDA determined that the VenoValve will meet an unmet clinical need, and our clinical trial negotiations to obtain our Investigational Device Exemption (IDE), as well as during our PMA submission interactions, we have established a productive and collaborative working relationship with the FDA over the past several years. We view this supervisory appeal as an opportunity to extend that relationship," said Robert Berman, enVVeno Medical's Chief Executive Officer. "Bringing a true first-in-class device through the PMA regulatory process raises unique challenges, and it is not unusual to have sequential collaborative discussions with the Agency to address issues that arise during the review process. We are committed to our continuing interactions with the FDA and to the goal of bringing the VenoValve to the 2.5 to 3.5 million patients suffering from severe deep venous CVI in the U.S. and who have no effective treatment options."
Internal Agency reviews are based on information already in the administrative file. Due to the variety of both physician reported and patient reported data generated by the VenoValve pivotal study and which is already a part of the file, the Company is confident that explaining this data to supervisory management in a focused appeal setting will lead to a positive outcome, with a decision expected by the end of 2025.
About enVVeno Medical Corporation
enVVeno Medical (NASDAQ: NVNO) is an Irvine, California-based, late clinical-stage medical device Company focused on the advancement of innovative bioprosthetic (tissue-based) solutions to improve the standard of care for the treatment of deep venous disease. The Company's lead product, the VenoValve®, is a first-in-class surgical replacement venous valve being developed for the treatment of severe deep Chronic Venous Insufficiency (CVI). The Company is also developing a non-surgical, transcatheter based replacement venous valve for the treatment of deep venous CVI called enVVe®. Both the VenoValve and enVVe are designed to act as one-way valves, to help assist in propelling blood up the leg, and back to the heart and lungs. The Company is currently performing the final testing necessary to seek IDE approval from the FDA to begin the U.S. pivotal trial for enVVe.
Cautionary Note on Forward-Looking Statements
This press release and any statements of stockholders, directors, employees, representatives and partners of enVVeno Medical Corporation (the "Company") related thereto contain, or may contain, among other things, certain "forward-looking statements" within the meaning of the Private Securities Litigation Reform Act of 1995. Such forward-looking statements involve significant risks and uncertainties. Such statements may include, without limitation, statements identified by words such as "projects," "may," "will," "could," "would," "should," "believes," "expects," "anticipates," "estimates," "intends," "plans," "potential" or similar expressions. These statements are based upon the current beliefs and expectations of the Company's management and are subject to significant risks and uncertainties, including, but not limited to that such FDA appeal is unsuccessful and other risks detailed in the Company's filings with the Securities and Exchange Commission. Actual results and timing may differ significantly from those set forth or implied in the forward-looking statements. Forward-looking statements involve certain risks and uncertainties that are subject to change based on various factors (many of which are beyond the Company's control). The Company undertakes no obligation to publicly update any forward-looking statements, whether as a result of new information, future presentations or otherwise, except as required by applicable law.
INVESTOR CONTACT:
Jenene Thomas, JTC Team, LLC
[email protected]
(908) 824-0775
MEDIA CONTACT:
Glenn Silver, FINN Partners
[email protected]
(973) 818-8198
SOURCE: enVVeno Medical Corporation
View the original press release on ACCESS Newswire
J.Williams--AMWN