
-
 Rodman inks record-setting contract with NWSL'S Spirit
Rodman inks record-setting contract with NWSL'S Spirit
-
TikTok establishes joint venture to end US ban threat

-
 Dodgers' latest splurge reignites baseball salary cap debate
Dodgers' latest splurge reignites baseball salary cap debate
-
Iran warns 'finger on trigger' as Trump says Tehran wants talks

-
 'Basic tennis etiquette' - Navratilova, Davenport condemn Osaka
'Basic tennis etiquette' - Navratilova, Davenport condemn Osaka
-
Fuming Kyrgios 'does not know' what comes after Australian Open

-
 Arsenal face Man Utd test as City search for spark
Arsenal face Man Utd test as City search for spark
-
'Vigilant' Europe eyes next Trump shock after Greenland climbdown

-
 Workers dig for the missing in New Zealand landslide
Workers dig for the missing in New Zealand landslide
-
Scheffler tied for second behind Lee, Coody in La Quinta

-
 Patriots vie for Super Bowl return against Broncos
Patriots vie for Super Bowl return against Broncos
-
Arctic blast to wallop N. America -- is climate change to blame?

-
 Trump bruised hand on table, he says of new photos
Trump bruised hand on table, he says of new photos
-
NYC sues to block Dr. Phil-fronted police TV show

-
 Intel shares plunge on earnings expectations
Intel shares plunge on earnings expectations
-
Villa seal place in Europa League last 16 as Forest beaten

-
 White House X account alters protester photo to add tears
White House X account alters protester photo to add tears
-
US negotiators meet Putin for high-stakes Ukraine talks

-
 US stocks rally again after Trump backs off Greenland tariff threat
US stocks rally again after Trump backs off Greenland tariff threat
-
Ecuador, Colombia ramp up trade war with tit-for-tat energy levies

-
 Trump bruised hand on table, White House says of new photos
Trump bruised hand on table, White House says of new photos
-
Japan PM Takaichi set to dissolve parliament for snap election

-
 Carney answers Trump: 'Canada doesn't live because of US'
Carney answers Trump: 'Canada doesn't live because of US'
-
Trump pitches Miami for World Expo 2035

-
 Venezuela moves to open up oil sector, a key Trump demand
Venezuela moves to open up oil sector, a key Trump demand
-
Trump sues JPMorgan Chase, CEO Dimon, claims 'debanked' for politics

-
 Chile police arrest third suspect in wildfire-ravaged south
Chile police arrest third suspect in wildfire-ravaged south
-
Galthie confirms Dupont as France captain for Six Nations

-
 Villa seal place in Europa League last 16 as Celtic draw in Italy
Villa seal place in Europa League last 16 as Celtic draw in Italy
-
Musk's Grok created three million sexualized images, research says

-
 Gazans pay homage to Palestinian journalists killed by Israel
Gazans pay homage to Palestinian journalists killed by Israel
-
With 'Board of Peace,' Trump tries hand at institution-making, to wide doubt

-
 At Davos, Zelensky blasts EU, says US 'security guarantees' ready
At Davos, Zelensky blasts EU, says US 'security guarantees' ready
-
French navy boards tanker 'from Russia' in Mediterranean

-
 Trump takes Davos on wild ride
Trump takes Davos on wild ride
-
Venezuela moves to liberalize oil sector, in boost for Trump

-
 Venezuela looks to petrodollars to bring down prices
Venezuela looks to petrodollars to bring down prices
-
Europe relieved but 'vigilant' after Trump Greenland climbdown

-
 Freezing Kyiv residents seek warmth in trains and tents
Freezing Kyiv residents seek warmth in trains and tents
-
Musk makes Davos debut with promise of robots for all

-
 Track star Sydney McLaughlin-Levrone announces pregnancy
Track star Sydney McLaughlin-Levrone announces pregnancy
-
NYC sues to block Dr. Phil-fronted police documentary

-
 Basking in Oscar nod, Russian videographer ready for Hollywood
Basking in Oscar nod, Russian videographer ready for Hollywood
-
WTO chief slams rise of trade protectionism

-
 Sri Lanka seal 19-run win over England in opening ODI
Sri Lanka seal 19-run win over England in opening ODI
-
Germany expels Russian alleged spy handler, Moscow vows response

-
 Casemiro to leave Man Utd at end of season
Casemiro to leave Man Utd at end of season
-
Frank says troubled Spurs 'going in right direction'

-
 Springboks to meet All Blacks in USA for first time
Springboks to meet All Blacks in USA for first time
-
Men's fashion turns to embroidery as guys want 'something different’


ClearPoint Neuro Announces EU MDR Certification for ClearPoint Navigation Software Version 3.0.2, Expanding Access to the Latest Operating Room Navigation Platform in Europe
SOLANA BEACH, CALIFORNIA / ACCESS Newswire / January 22, 2026 / ClearPoint Neuro, Inc. (NASDAQ:CLPT) (the "Company"), a global device, cell, and gene therapy-enabling company offering precise navigation to the brain and spine, today announced it has received EU MDR Certification for its ClearPoint Navigation Software Version 3.0.2.
"By achieving CE Mark for the ClearPoint Navigation 3.0.2 software, we are able to unify our global navigation platform which we believe will enable consistent training and hospital IT support," commented Mazin Sabra, Chief Operating Officer at ClearPoint Neuro. "We expect that this will also help us to not only satisfy our biopharma partners who want a global solution, but also reduce our operating costs and drive economies of scale."
ClearPoint Navigation Software Version 3.x was first introduced in the United States following FDA clearance 11 months ago and has already been adopted by most US customers. "By releasing our latest version of software to our EU customers, we are excited to offer feature refinements developed through years of valuable user experience and feedback," stated Tim Orr, VP of Software Development at ClearPoint Neuro. "The 3.0.2 release represents an important milestone which will allow us to unify US and EU customers on the same navigation platform."
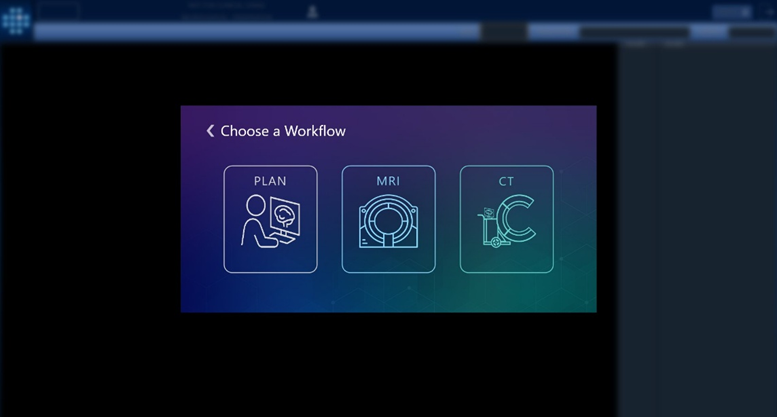
Version 3.0.2, now available in Europe, introduces an intraoperative CT workflow that builds on over a decade of experience in stereotactic procedures. While earlier generations of ClearPoint software supported MRI-guided workflows exclusively, the 3.0.2 release extends ClearPoint navigation capabilities to the operating room. By offering compatibility with both intraoperative CT and Cone-beam CT imaging, the software is designed to increase access to precision-guided neurosurgery for facilities without intraoperative MRI capabilities. The ClearPoint Navigation Software Version 3.0.2, when used in conjunction with the SmartFrame XG stereotactic frame, is intended to provide precise stereotactic guidance when placing instruments or devices during neurosurgical procedures. These procedures include biopsies, catheter and electrode insertion including deep brain stimulation (asleep or awake) lead placement.
About ClearPoint Neuro
ClearPoint Neuro is a device, cell, and gene therapy-enabling company offering precise navigation to the brain and spine. The Company uniquely provides both established clinical products as well as pre-clinical development services for controlled drug and device delivery. The Company's flagship product, the ClearPoint Neuro Navigation System, has FDA clearance and is CE-marked. ClearPoint Neuro is engaged with healthcare and research centers in North America, Europe, Asia, and South America. The Company is also partnered with the most innovative pharmaceutical/biotech companies, academic centers, and contract research organizations, providing solutions for direct CNS delivery of therapeutics in pre-clinical studies and clinical trials worldwide. To date, thousands of procedures have been performed and supported by the Company's field-based clinical specialist team, which offers support and services to our customers and partners worldwide. For more information, please visit www.clearpointneuro.com.
Forward-Looking Statements
This press release contains forward-looking statements within the context of the federal securities laws, including the Company's expectation for the future market of its products and services, expectations for reducing costs and gaining efficiencies by enablement of a unified software platform, and other performance and results. These forward-looking statements are based on management's current expectations and are subject to the risks inherent in the business, which may cause the Company's actual results to differ materially from those expressed in or implied by forward-looking statements. Particular uncertainties and risks include those relating to: global and political instability; geopolitical trends, such as protectionism and economic nationalism; the introduction of or changes in tariffs, sanctions, or trade barriers; supply chain disruptions and macroeconomic and inflationary conditions; future revenue from sales of the Company's products and services; the Company's ability to market, commercialize and achieve broader market acceptance for new products and services offered by the Company; the ability of our biologics and drug delivery partners to achieve commercial success, including their use of the Company's products and services in their delivery of therapies; the Company's expectations, projections and estimates regarding expenses, future revenue, capital requirements, and the availability of and the need for additional financing; the Company's ability to obtain additional funding to support its research and development programs; the ability of the Company to manage the growth of its business; the Company's ability to attract and retain its key employees; and risks inherent in the research, development, and regulatory approval of new products. For a detailed description of the Company's risks and uncertainties, you are encouraged to review its documents filed with the SEC including the Company's recent filings on Form 8-K, Form 10-K and Form 10-Q. You are cautioned not to place undue reliance on forward-looking statements, which speak only as of the date on which they were made. The Company undertakes no obligation to update such statements to reflect events that occur or circumstances that exist after the date on which they were made, except as required by law.
Contact:
Media Contact:
[email protected]
Investor Relations:
Danilo D'Alessandro, Chief Financial Officer
(888) 287-9109
[email protected]
SOURCE: ClearPoint Neuro, Inc.
View the original press release on ACCESS Newswire
L.Durand--AMWN


