-
 Bridgeman powers to six-shot lead over McIlroy at Riviera
Bridgeman powers to six-shot lead over McIlroy at Riviera
-
Artist creates 'Latin American Mona Lisa' with plastic bottle caps
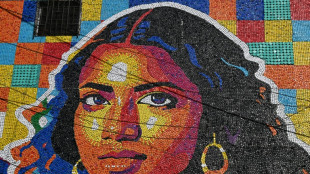
-
 Malinin highlights mental health as Shaidorov wears panda suit at Olympic skating gala
Malinin highlights mental health as Shaidorov wears panda suit at Olympic skating gala
-
Timberwolves center Gobert suspended after another flagrant foul

-
 Guardiola hails Man City's 'massive' win over Newcastle
Guardiola hails Man City's 'massive' win over Newcastle
-
PSG win to reclaim Ligue 1 lead after Lens lose to Monaco

-
 Man City down Newcastle to pile pressure on Arsenal, Chelsea held
Man City down Newcastle to pile pressure on Arsenal, Chelsea held
-
Man City close gap on Arsenal after O'Reilly sinks Newcastle

-
 Finland down Slovakia to claim bronze in men's ice hockey
Finland down Slovakia to claim bronze in men's ice hockey
-
More than 1,500 request amnesty under new Venezuela law

-
 US salsa legend Willie Colon dead at 75
US salsa legend Willie Colon dead at 75
-
Canada beat Britain to win fourth Olympic men's curling gold

-
 Fly-half Jalibert ruled out of France side to face Italy
Fly-half Jalibert ruled out of France side to face Italy
-
Russell restart try 'big moment' in Scotland win, says Townsend

-
 Kane helps Bayern extend Bundesliga lead as Dortmund held by Leipzig
Kane helps Bayern extend Bundesliga lead as Dortmund held by Leipzig
-
Liga leaders Real Madrid stung by late Osasuna winner

-
 Ilker Catak's 'Yellow Letters' wins Golden Bear at Berlin film festival
Ilker Catak's 'Yellow Letters' wins Golden Bear at Berlin film festival
-
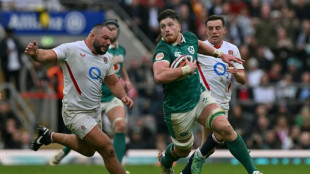
England's Genge says thumping Six Nations loss to Ireland exposes 'scar tissue'
-
 Thousands march in France for slain far-right activist
Thousands march in France for slain far-right activist
-
Imperious Alcaraz storms to Qatar Open title

-
 Klaebo makes Olympic history as Gu forced to wait
Klaebo makes Olympic history as Gu forced to wait
-
Late Scotland try breaks Welsh hearts in Six Nations

-
 Lens lose, giving PSG chance to reclaim Ligue 1 lead
Lens lose, giving PSG chance to reclaim Ligue 1 lead
-
FIFA's Gaza support 'in keeping' with international federation - IOC

-
 First all-Pakistani production makes history at Berlin film fest
First all-Pakistani production makes history at Berlin film fest
-
Gu forced to wait as heavy snow postpones Olympic halfpipe final

-
 NASA chief rules out March launch of Moon mission over technical issues
NASA chief rules out March launch of Moon mission over technical issues
-
Dutch double as Bergsma and Groenewoud win Olympic speed skating gold

-
 At least three dead as migrant boat capsizes off Greek island
At least three dead as migrant boat capsizes off Greek island
-
Struggling Juventus' woes deepen with home loss to Como

-
 Chelsea, Aston Villa held in blow to Champions League hopes
Chelsea, Aston Villa held in blow to Champions League hopes
-
Thousands march in France for slain far-right activist under heavy security

-
 Kane nets double as Bundesliga leaders Bayern beat Frankfurt
Kane nets double as Bundesliga leaders Bayern beat Frankfurt
-
Canada beat USA to take bronze in Olympic women's curling

-
 Hunger and belief key to Ireland's win, says Sheehan
Hunger and belief key to Ireland's win, says Sheehan
-
Pegula sees off Svitolina to win Dubai WTA 1000 title

-
 Trump hikes US global tariff rate to 15%
Trump hikes US global tariff rate to 15%
-
AI revolution looms over Berlin film fest

-
 Gibson-Park guides Ireland to record-breaking win in England
Gibson-Park guides Ireland to record-breaking win in England
-
Defence the priority for France against Italy, says Dupont

-
 Juventus end bad week with 2-0 loss against Como
Juventus end bad week with 2-0 loss against Como
-
Libya's Ramadan celebrations tempered by economic woes

-
 Norway's cross-country king Klaebo wins sixth gold of Milan-Cortina Winter Olympics
Norway's cross-country king Klaebo wins sixth gold of Milan-Cortina Winter Olympics
-
Iranian students chant anti-government slogans, as US threats loom

-
 Hezbollah vows resistance after deadly Israeli strike
Hezbollah vows resistance after deadly Israeli strike
-
'Stormy seas' of Gaza row overshadow Berlin film fest finale

-
 Pakistan-New Zealand Super Eights clash delayed by rain
Pakistan-New Zealand Super Eights clash delayed by rain
-
Werder Bremen cancel US tour citing 'political reasons'

-
 South Africa's De Kock says handling pressure key in India clash
South Africa's De Kock says handling pressure key in India clash
-
French volunteer bakes for Ukraine amid frosts and power outages


Regentis Biomaterials Appoints Ori Gon as Chief Financial Officer and Chief Business Officer as GelrinC Progresses Towards Commercial Launch in Europe and Advances in U.S. Phase III FDA Trial
GelrinC, the only restorative product for knee cartilage repair, has CE Mark approval in Europe and is currently at the midpoint of a pivotal FDA Phase III trial in the U.S.
Ori Gon brings substantial public company, medtech, and capital markets experience
HERZLIYA, ISRAEL / ACCESS Newswire / February 4, 2026 / Regentis Biomaterials Ltd., ("Regentis" or the "Company") (NYSE American:RGNT), a regenerative medicine company focused on innovative tissue repair solutions, today announced the appointment of Ori Gon as Chief Financial Officer and Chief Business Officer, effective immediately. Mr. Gon will lead the Company's commercial and business development activities, as well as its financial strategy, planning, and reporting.
Mr. Gon joins Regentis at a pivotal time as GelrinC®, the Company's proprietary hydrogel implant for knee cartilage repair, advances towards commercial launch in Europe following CE Mark approval and is being evaluated in a pivotal Phase III FDA clinical trial in the United States.
"Ori's appointment significantly strengthens our leadership team as we transition from a development-stage company toward commercialization," said Dr. Ehud Geller, Executive Chairman of Regentis. "His deep experience as a public company CFO, combined with his strategic business development expertise, will be instrumental as we evaluate strategic partnerships to launch GelrinC® in Europe and build the foundation for sustainable, revenue-generating growth."
Mr. Gon brings over 15 years of financial leadership experience across public and private enterprises and medical technology. Most recently, he served as CFO at Tactile Mobility, a sensing and data analytics company focused on advanced automotive and mobility applications. Prior to that, he held senior financial leadership roles at ReWalk Robotics, Inc., now Nasdaq-listed Lifeward Ltd., a pioneer in wearable robotic exoskeletons for individuals with lower limb disabilities, where he served as CFO and Corporate Controller. Earlier in his career, he was Controller at On Track Innovations Ltd., a Nasdaq- and Neuer Markt-listed fintech company. He began his professional career as an auditor at KPMG Israel.
Mr. Gon has led multiple secondary public offerings and financing transactions across various structures, raising over $150 million in aggregate capital. He is a Certified Public Accountant (CPA) in Israel.
"I am excited to join Regentis at such an important inflection point," said Ori Gon. "With GelrinC® approaching commercialization in Europe and progressing through a pivotal FDA trial in the U.S., we believe Regentis is uniquely positioned to transform the treatment landscape for knee cartilage repair. I look forward to working with the team to execute our commercial strategy, build strategic partnerships, and create long-term value for patients and stakeholders."
About GelrinC®
Regentis' lead product, GelrinC®, is a cell-free, off-the-shelf hydrogel synchronized erosion and resorbable implant for the treatment of painful injuries to focal articular knee cartilage. As an innovative regenerative medical product, GelrinC® offers an unprecedented solution that gives surgeons and payers an off-the-shelf, ready to use, simple to perform, reliable, and cost-effective procedure that provides patients with a single, 10-minute procedure, faster recovery, sustained pain relief, and functional improvement for more than 4 years, based on clinical study results to date. No effective off-the-shelf, ready to use treatment for focal knee cartilage defects is currently available on the market. GelrinC® has CE Mark approval in the European Union and is now being evaluated in a pivotal U.S. Food and Drug Administration (FDA) study, which has completed over 50% enrollment.
About Regentis Biomaterials
Regentis Biomaterials Ltd is a regenerative medicine company dedicated to developing innovative tissue repair solutions that restore health and enhance quality of life. With an initial focus on orthopedic treatments, Regentis' Gelrin platform technology, based on synchronized, degradable hydrogel implants, regenerates damaged or diseased tissue including inflamed cartilage and bone. Regentis' lead product GelrinC®, is a cell-free, off-the-shelf hydrogel that is eroded and resorbed in the knee, allowing the surrounding cells to regenerate the cartilage in a controlled and synchronous process. GelrinC® aims to address a market of approximately 470,000 cases for cartilage knee repair annually in the U.S. where no off-the-shelf treatment is available.
Forward Looking Statements
This press release contains "forward-looking statements" that are subject to substantial risks and uncertainties. All statements, other than statements of historical fact, contained in this press release are forward-looking statements. Forward-looking statements contained in this press release may be identified by the use of words such as "anticipate," "believe," "contemplate," "could," "estimate," "expect," "intend," "seek," "may," "might," "plan," "potential," "predict," "project," "target," "aim," "should," "will" "would," or the negative of these words or other similar expressions, although not all forward-looking statements contain these words, and include beliefs regarding Regentis' advancement towards commercialization. Forward-looking statements are based on Regentis' current expectations and are subject to inherent uncertainties, risks and assumptions that are difficult to predict. Further, certain forward-looking statements are based on assumptions as to future events that may not prove to be accurate. Factors that may affect future results and may cause these forward-looking statements to be inaccurate include, without limitation: the ability of our clinical trials to demonstrate safety and efficacy of GelrinC or any future product candidate, and other positive results; the timing and focus of our preclinical studies and clinical trials, and the reporting of data from those studies and trials; the size of the market opportunity for of GelrinC or any future product candidate, including our estimates of the number of patients who suffer from the diseases we are targeting; our ability to accurately identify demand for product candidates; the success of competing therapies that are or may become available; the beneficial characteristics, safety, efficacy and therapeutic effects of our product candidates; our ability to obtain FDA approval for of GelrinC or any future product candidate and obtain and maintain regulatory approval; our ability to obtain market acceptance of of GelrinC or any future product candidate from the medical community and third-party payors; our plans relating to the further development of GelrinC or any future product candidate, including additional disease states or indications we may pursue; existing regulations and regulatory developments in the United States and other jurisdictions; our plans and ability to obtain or protect intellectual property rights, including extensions of patent terms where available and our ability to avoid infringing the intellectual property rights of others; the need to hire additional personnel and our ability to attract and retain such personnel; our estimates regarding expenses, future revenue, capital requirements and needs for additional financing; our dependence on third parties; our financial performance and our ability to repay our loans and debts; and our ability to negotiate favorable terms in any collaboration, licensing or other arrangements into which we may enter and perform our obligations under such collaborations. For a more detailed description of the risks and uncertainties affecting Regentis, reference is made to the Company's reports filed from time to time with the Securities and Exchange Commission ("SEC"), including, but not limited to, the risks detailed in the section titled "Risk Factors" in the final prospectus related to the public offering filed with the SEC. Forward-looking statements contained in this announcement are made as of this date, and Regentis undertakes no duty to update such information except as required under applicable law.
Contact:
SOURCE: Regentis Biomaterials Ltd
View the original press release on ACCESS Newswire
B.Finley--AMWN