
-
 Sinner eases into Italian Open last 16, Osaka dumped out
Sinner eases into Italian Open last 16, Osaka dumped out
-
Real Madrid duo Vinicius, Vazquez injured

-
 Indian PM Modi vows strong response to any future 'terrorist attack'
Indian PM Modi vows strong response to any future 'terrorist attack'
-
Opening statements start in Sean 'Diddy' Combs trial

-
 Snow cover of Swiss glaciers below average this year: study
Snow cover of Swiss glaciers below average this year: study
-
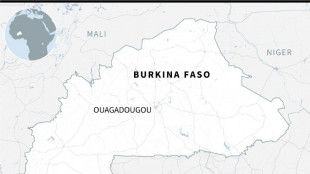
Jihadist attack kills 'several dozen' in Burkina Faso
-
 Ancelotti to leave Real Madrid for Brazil job
Ancelotti to leave Real Madrid for Brazil job
-
Trump announces drug prices cut with swipe at Europe

-
 Ancelotti exits Madrid, hoping to add World Cup with Brazil
Ancelotti exits Madrid, hoping to add World Cup with Brazil
-
US, China agree to slash tariffs as Trump says to speak with Xi soon

-
 Ancelotti to take over as Brazil coach
Ancelotti to take over as Brazil coach
-
Israel urges ICC to drop arrest warrants against PM

-
 Poland to close Russian consulate in Krakow over 'sabotage'
Poland to close Russian consulate in Krakow over 'sabotage'
-
Kremlin rejects Europe's 'ultimatums' for truce with Ukraine

-
 Ireland rugby captain Doris ruled out for up to six months
Ireland rugby captain Doris ruled out for up to six months
-
Algerian attack survivor vows to be heard in court battle with award-winning author

-
 Europa League glory could be 'turning point' for Spurs: Postecoglou
Europa League glory could be 'turning point' for Spurs: Postecoglou
-
White S.Africans resettled in US did not face 'persecution': govt

-
 Gaza faces 'critical risk of famine': UN report
Gaza faces 'critical risk of famine': UN report
-
Indian teams defuse bombs in Kashmir border areas

-
 Kim Kardashian testifies in Paris multi-million-dollar robbery trial
Kim Kardashian testifies in Paris multi-million-dollar robbery trial
-
Alexander-Arnold exit will not overshadow Liverpool title party: Van Dijk

-
 Osaka knocked out of Italian Open as fans await Sinner
Osaka knocked out of Italian Open as fans await Sinner
-
France condemns 'fake news' over Europe leaders' cocaine accusation

-
 Indian PM Modi set to address nation after Pakistan truce
Indian PM Modi set to address nation after Pakistan truce
-
With Israel ties on the table, UAE offers Saudis an example

-
 UK urges Putin to 'get serious about peace'
UK urges Putin to 'get serious about peace'
-
Leicester Tigers name Parling to replace Cheika as head coach

-
 UK govt toughens immigration plans as hard-right gains
UK govt toughens immigration plans as hard-right gains
-
Markets rally after China, US slash tariffs

-
 Leo XIV urges release of jailed journalists as Zelensky invites to Ukraine
Leo XIV urges release of jailed journalists as Zelensky invites to Ukraine
-
Film legend Bardot backs Depardieu ahead of sexual assault verdict

-
 Mbappe shows fallen Real Madrid new road to riches
Mbappe shows fallen Real Madrid new road to riches
-
Drones hit Ukraine as Zelensky awaits Putin reply on talks

-
 Indian great Kohli follows Rohit in retiring from Test cricket
Indian great Kohli follows Rohit in retiring from Test cricket
-
UK hosts European ministers for Ukraine talks amid ceasefire call

-
 Copenhagen to offer giveaways to eco-friendly tourists
Copenhagen to offer giveaways to eco-friendly tourists
-
Ocalan: founder of the Kurdish militant PKK who authored its end

-
 Kurdish militant PKK says disbanding, ending armed struggle
Kurdish militant PKK says disbanding, ending armed struggle
-
Under pressure, UK govt unveils flagship immigration plans

-
 India great Virat Kohli retires from Test cricket
India great Virat Kohli retires from Test cricket
-
US, China agree to slash tariffs in trade war de-escalation

-
 Markets rally after China and US slash tariffs for 90 days
Markets rally after China and US slash tariffs for 90 days
-
India, Pakistan military to confer as ceasefire holds

-
 Kurdish militant group PKK says disbanding, ending armed struggle
Kurdish militant group PKK says disbanding, ending armed struggle
-
Virat Kohli: Indian batting great and hero to hundreds of millions

-
 India great Virat Kohli announces retirement from Test cricket
India great Virat Kohli announces retirement from Test cricket
-
Netanyahu vows further fighting despite planned US-Israeli hostage release

-
 Salt of the earth: Pilot project helping reclaim Sri Lankan farms
Salt of the earth: Pilot project helping reclaim Sri Lankan farms
-
UK towns harness nature to combat rising flood risk


Ensysce Biosciences Bolsters Management Team with Regulatory Expert
~ Advanced Preparation for New Drug Application ~
SAN DIEGO, CA / ACCESS Newswire / May 12, 2025 / Ensysce Biosciences, Inc. (NASDAQ:ENSC) ("Ensysce" or the "Company"), a clinical-stage pharmaceutical company developing innovative solutions for severe pain relief while reducing the potential for abuse and overdose, today announced it has added Tracy Hysong, CCRA as Senior Director of Regulatory Affairs to its management team.
Ms. Hysong is a Certified Clinical Research Associate (CCRA) through the Association of Clinical Research Professionals with years of experience undertaking regulatory activity at the University of California Davis (UC Davis). She helped establish the UC Davis Clinical Trials Office as part of the Clinical and Translational Science Center (CTSC) at UC Davis Health. Her expertise will bolster the regulatory team at Ensysce as the Company focuses on the execution of multiple clinical trials. Over the next year, Ensysce will be initiating its Phase 3 pivotal study to evaluate the efficacy of PF614 and develop its New Drug Application (NDA) for submission in 2026. Additionally, Ensysce has ongoing clinical studies to evaluate a groundbreaking overdose-protected pain medicine, PF614-MPAR, and is preparing for an Investigational New Drug (IND) Application and Phase 1 study of its novel treatment for opioid use disorder (OUD).
Dr. Lynn Kirkpatrick, CEO of Ensysce, added, "The ability to bring Tracy on board at this time is crucial for the Company as it handles three current INDs, prepares to submit a fourth IND for PF9001 for OUD, and plans the regulatory submission of an NDA for PF614. Tracy's experience with clinical trial support and regulatory affairs is perfectly timed to meet the Company's needs going forward. As recently reported, we believe our highly novel treatment of OUD, with both abuse deterrence and overdose protection built in, will be moving through IND-enabling studies and IND submission in the coming year, in addition to the parallel regulatory activities associated with PF614 and PF614-MPAR. We welcome this addition of Tracy to our team."
About Ensysce Biosciences
Ensysce Biosciences is a clinical stage company with a goal of disrupting the analgesic landscape by introducing a new class of highly novel opioids for the treatment of severe pain. Leveraging its Trypsin-Activated Abuse Protection (TAAPTM) and Multi-Pill Abuse Resistance (MPAR®) platforms, the Company is developing unique, tamper-proof treatment options for pain that minimize the risk of both drug abuse and overdose. Ensysce's products are anticipated to provide safer options to treat patients suffering from severe pain and assist in preventing deaths caused by medication abuse. For more information, please visit www.ensysce.com.
Forward-Looking Statements
Statements contained in this press release that are not purely historical may be deemed to be forward-looking statements for the purposes of the safe harbor provisions under The Private Securities Litigation Reform Act of 1995 and other federal securities laws. Without limiting the foregoing, the use of words such as "may," "intends," "can," "might," "will," "expect," "plan," "possible," "believe" and other similar expressions are intended to identify forward-looking statements. The product candidates discussed are in clinic and not approved and there can be no assurance that the clinical programs will be successful in demonstrating safety and/or efficacy, that Ensysce will not encounter problems or delays in clinical development, or that any product candidate will ever receive regulatory approval or be successfully commercialized. All forward-looking statements are based on estimates and assumptions by Ensysce's management that, although Ensysce believes to be reasonable, are inherently uncertain. All forward-looking statements are subject to risks and uncertainties that may cause actual results to differ materially from those that Ensysce expected. In addition, Ensysce's business is subject to additional risks and uncertainties, including among others, the initiation and conduct of preclinical studies and clinical trials; the timing and availability of data from preclinical studies and clinical trials; expectations for regulatory submissions and approvals; potential safety concerns related to, or efficacy of, Ensysce's product candidates; the availability or commercial potential of product candidates; the ability of Ensysce to fund its continued operations, including its planned clinical trials; the dilutive effect of stock issuances from our fundraising; and Ensysce's and its partners' ability to perform under their license, collaboration and manufacturing arrangements. These statements are also subject to a number of material risks and uncertainties that are described in Ensysce's most recent quarterly report on Form 10-K and current reports on Form 8-K, which are available, free of charge, at the SEC's website at www.sec.gov. Any forward-looking statement speaks only as of the date on which it was made. Ensysce undertakes no obligation to publicly update or revise any forward-looking statement, whether as a result of new information, future events or otherwise, except as required under applicable law.
Ensysce Biosciences Company Contact:
Lynn Kirkpatrick, Ph.D.
Chief Executive Officer
(858) 263-4196
Ensysce Biosciences Investor Relations Contact:
Shannon Devine
MZ North America
Main: 203-741-8811
[email protected]
SOURCE: Ensysce Biosciences Inc.
View the original press release on ACCESS Newswire
S.Gregor--AMWN