
-
 Bitcoin hits record high amid optimism over US legislation
Bitcoin hits record high amid optimism over US legislation
-
'Tush push' survives as NFL ban fails to pass - reports

-
 NFL LA Games decision is flag football's 'Dream Team' moment: president
NFL LA Games decision is flag football's 'Dream Team' moment: president
-
Dollar, US bonds under pressure as Trump pushes tax bill

-
 London to host Laver Cup in 2026
London to host Laver Cup in 2026
-
LGBTQ Thai ghost story turns political in Cannes

-
 Carapaz wins stage 11 of Giro with Del Toro in lead
Carapaz wins stage 11 of Giro with Del Toro in lead
-
S.Africa's Ramaphosa woos Trump, Musk after tensions

-
 Teeth hurt? It could be because of a 500-million-year-old fish
Teeth hurt? It could be because of a 500-million-year-old fish
-
Third time lucky? South Africa presents revised budget

-
 Dollar, US bonds under pressure amid global tensions and Trump tax bill
Dollar, US bonds under pressure amid global tensions and Trump tax bill
-
French prosecutors urge 10-year terms for key accused in Kardashian theft

-
 Israeli 'warning' fire at diplomats sparks outcry amid Gaza pressure
Israeli 'warning' fire at diplomats sparks outcry amid Gaza pressure
-
Lyon hotshot Cherki called up by France for Nations League

-
 Stokes sets England's sights on getting to No 1 in Test rankings
Stokes sets England's sights on getting to No 1 in Test rankings
-
'Recovered' Assange promotes Cannes documentary wearing Gaza T-shirt

-
 England's Archer out of West Indies series in latest injury setback
England's Archer out of West Indies series in latest injury setback
-
Tiny Elversberg chasing Bundesliga promotion 'dream'

-
 Pro-Russia ex-Ukraine MP shot dead near Madrid
Pro-Russia ex-Ukraine MP shot dead near Madrid
-
Euro 2028 hosts must qualify but two places reserved for them

-
 'Recovered' Assange promotes Cannes documentary about his life
'Recovered' Assange promotes Cannes documentary about his life
-
'Maestro' Jalibert holds keys to Bordeaux-Begles' Champions Cup hopes

-
 Contenders lining up, eyeing Swiatek's French Open crown
Contenders lining up, eyeing Swiatek's French Open crown
-
Trump Organization breaks ground on $1.5-bn Vietnam project

-
 'Man to beat' Alcaraz wary of sharper Sinner at French Open
'Man to beat' Alcaraz wary of sharper Sinner at French Open
-
Bloomberg financial markets data service hit by outage
-
 EU plans to slash red tape for medium-sized companies
EU plans to slash red tape for medium-sized companies
-
Kremlin denies dragging out Ukraine peace talks

-
 Man Utd and Spurs face season-defining Europa League duel
Man Utd and Spurs face season-defining Europa League duel
-
Vietnam jails 23 people over rare earths exploitation

-
 Pepe Reina to play final match in Como's clash with Inter
Pepe Reina to play final match in Como's clash with Inter
-
Spike Lee says expensive for music artists to speak out

-
 China's Baidu posts rise in Q1 revenue as seeks to grow AI presence
China's Baidu posts rise in Q1 revenue as seeks to grow AI presence
-
Canal+ buyout of S.Africa's MultiChoice one step closer

-
 Pakistan drop stars Shaheen, Azam and Rizwan for Bangladesh T20s
Pakistan drop stars Shaheen, Azam and Rizwan for Bangladesh T20s
-
Australian ex-tennis star Dokic says estranged father dead

-
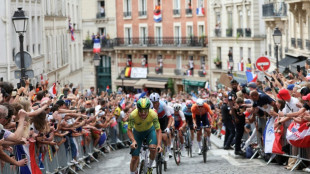 2025 Tour de France adds Montmartre suspense to final stage
2025 Tour de France adds Montmartre suspense to final stage
-
Trump Jr says 'maybe one day' he'll run for US president

-
 Gazans wait for aid as Israel faces mounting pressure
Gazans wait for aid as Israel faces mounting pressure
-
Macron party backs banning hijab in public spaces for under 15s

-
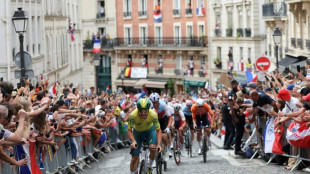 2025 Tour de France to include Montmartre on final stage: organisers
2025 Tour de France to include Montmartre on final stage: organisers
-
French prosecutors urge 10-year term for alleged Kardashian theft ringleader

-
 Guardiola warns he'll quit if Man City squad too large
Guardiola warns he'll quit if Man City squad too large
-
Cyberattack costs UK retailer Marks & Spencer £300 mn

-
 Six killed in school bus bombing in SW Pakistan
Six killed in school bus bombing in SW Pakistan
-
India's lion population rises by a third

-
 UK inflation hits 15-month high as utility bills soar
UK inflation hits 15-month high as utility bills soar
-
Oil prices jump on report of Israel prepping Iran strike

-
 British climbers summit Everest in record bid
British climbers summit Everest in record bid
-
China slams US 'bullying' over new warnings on Huawei chips

Moderna Provides Update on BLA Submission for Combination Vaccine Against Influenza and COVID-19
CAMBRIDGE, MA / ACCESS Newswire / May 21, 2025 / Moderna, Inc. (Nasdaq:MRNA), today announced that in consultation with the U.S. Food and Drug Administration (FDA), the Company has voluntarily withdrawn the pending Biologics License Application (BLA) for mRNA-1083, its flu/COVID combination vaccine candidate for adults aged 50 years and older. The Company plans to resubmit the BLA later this year, after vaccine efficacy data from the ongoing Phase 3 trial of its investigational seasonal influenza vaccine, mRNA-1010, are available. Moderna continues to expect interim data from the mRNA-1010 trial to be available this summer.
About Moderna
Moderna is a leader in the creation of the field of mRNA medicine. Through the advancement of mRNA technology, Moderna is reimagining how medicines are made and transforming how we treat and prevent disease for everyone. By working at the intersection of science, technology and health for more than a decade, the company has developed medicines at unprecedented speed and efficiency, including one of the earliest and most effective COVID-19 vaccines.
Moderna's mRNA platform has enabled the development of therapeutics and vaccines for infectious diseases, immuno-oncology, rare diseases and autoimmune diseases. With a unique culture and a global team driven by the Moderna values and mindsets to responsibly change the future of human health, Moderna strives to deliver the greatest possible impact to people through mRNA medicines. For more information about Moderna, please visit modernatx.com and connect with us on X (formerly Twitter), Facebook, Instagram, YouTube and LinkedIn.
Forward-Looking Statements
This press release contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995, as amended, including statements regarding: the withdrawal of the BLA for mRNA-1083, plans for future resubmission of the mRNa-1083 BLA, and anticipated timing for efficacy data for Moderna's seasonal flu vaccine candidate, mRNA-1010. The forward-looking statements in this press release are neither promises nor guarantees, and you should not place undue reliance on these forward-looking statements because they involve known and unknown risks, uncertainties, and other factors, many of which are beyond Moderna's control and which could cause actual results to differ materially from those expressed or implied by these forward-looking statements. These risks, uncertainties, and other factors include, among others, those risks and uncertainties described under the heading "Risk Factors" in Moderna's Annual Report on Form 10-K for the fiscal year ended December 31, 2024, and in subsequent filings made by Moderna with the U.S. Securities and Exchange Commission, which are available on the SEC's website at www.sec.gov. Except as required by law, Moderna disclaims any intention or responsibility for updating or revising any forward-looking statements contained in this press release in the event of new information, future developments or otherwise. These forward-looking statements are based on Moderna's current expectations and speak only as of the date of this press release.
Moderna Contacts
Media:
Chris Ridley
Global Head of Media Relations
+1 617-800-3651
[email protected]
Investors:
Lavina Talukdar
Senior Vice President & Head of Investor Relations
+1 617-209-5834
[email protected]
SOURCE: Moderna, Inc.
View the original press release on ACCESS Newswire
O.Johnson--AMWN
