
-
 US Supreme Court allows third country deportations to resume
US Supreme Court allows third country deportations to resume
-
Oil prices tumble as markets shrug off Iranian rebuttal to US

-
 Rishabh Pant: India's unorthodox hero with 'method to his madness'
Rishabh Pant: India's unorthodox hero with 'method to his madness'
-
PSG ease past Seattle Sounders and into Club World Cup last 16

-
 Atletico win in vain as Botafogo advance at Club World Cup
Atletico win in vain as Botafogo advance at Club World Cup
-
Osaka, Azarenka advance on grass at Bad Homburg

-
 Haliburton latest NBA star with severe injury in playoffs
Haliburton latest NBA star with severe injury in playoffs
-
Trump wants quick win in Iran, but goal remains elusive

-
 Iran attacks US base in Qatar, Trump says time to make peace
Iran attacks US base in Qatar, Trump says time to make peace
-
Kasatkina falls, Fonseca secures first win on grass at Eastbourne

-
 Iran attacks US base in Qatar in retaliation for strikes on nuclear sites
Iran attacks US base in Qatar in retaliation for strikes on nuclear sites
-
Club World Cup prize money does not mean more pressure: Chelsea boss Maresca

-
 Leeds sign Slovenia defender Bijol from Udinese
Leeds sign Slovenia defender Bijol from Udinese
-
E.coli can turn plastic into painkillers, chemists discover

-
 Bluff and last-minute orders: Trump's path to Iran decision
Bluff and last-minute orders: Trump's path to Iran decision
-
US strikes on Iran open rift in Trump's support base

-
 Indiana's Haliburton has torn right Achilles tendon: reports
Indiana's Haliburton has torn right Achilles tendon: reports
-
England rally after Pant heroics to set up thrilling finish to India opener

-
 US hit by first extreme heat wave of the year
US hit by first extreme heat wave of the year
-
Holders Thailand among seven set for LPGA International Crown

-
 England set 371 to win India series opener after Pant heroics
England set 371 to win India series opener after Pant heroics
-
UK and Ukraine agree to deepen ties as Zelensky meets Starmer

-
 New York state to build nuclear power plant
New York state to build nuclear power plant
-
Syria announces arrests over Damascus church attack

-
 Bradley eyes playing captain role at Ryder Cup after win
Bradley eyes playing captain role at Ryder Cup after win
-
US existing home sales little-changed on sluggish market

-
 Top US court takes case of Rastafarian whose hair was cut in prison
Top US court takes case of Rastafarian whose hair was cut in prison
-
Greece declares emergency on Chios over wildfires

-
 Embattled Thai PM reshuffles cabinet as crisis rages
Embattled Thai PM reshuffles cabinet as crisis rages
-
Killer whales spotted grooming each other with seaweed

-
 Where is Iran's uranium? Questions abound after US strikes
Where is Iran's uranium? Questions abound after US strikes
-
EU approves MotoGP takeover by F1 owner Liberty Media

-
 Duplantis says vaulting 6.40m is within the 'realm of possibility'
Duplantis says vaulting 6.40m is within the 'realm of possibility'
-
Pant piles on agony for England with record-breaking century

-
 NATO to take 'quantum leap' with 5% summit pledge: Rutte
NATO to take 'quantum leap' with 5% summit pledge: Rutte
-
Textor sells Crystal Palace stake to boost hopes of European competition

-
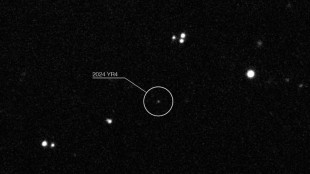 Earth's satellites at risk if asteroid smashes into Moon: study
Earth's satellites at risk if asteroid smashes into Moon: study
-
Syria president vows those involved in church attack will face justice

-
 Russian barrage kills 10 in Kyiv, including 11-year-old girl
Russian barrage kills 10 in Kyiv, including 11-year-old girl
-
Military bases or vital waterway: Iran weighs response to US strikes
-
 Italian sculptor Arnaldo Pomodoro dies aged nearly 99
Italian sculptor Arnaldo Pomodoro dies aged nearly 99
-
Rahul and Pant build India lead against England

-
 UK probes maternity services after scandals
UK probes maternity services after scandals
-
Asian countries most vulnerable to Strait of Hormuz blockade

-
 Anger as Kanye West to perform in Slovakia after Hitler song
Anger as Kanye West to perform in Slovakia after Hitler song
-
Israel targets Iran Guards, Tehran prison in fresh wave of strikes

-
 Star-packed, Covid-shaped 'Death Stranding 2' drops this week
Star-packed, Covid-shaped 'Death Stranding 2' drops this week
-
IOC is in 'best of hands', says Bach as he hands over to Coventry

-
 Oil prices seesaw as investors await Iran response to US strikes
Oil prices seesaw as investors await Iran response to US strikes
-
Beijing issues weather warning for hottest days of year


Telomir Pharmaceuticals Secures $3 Million at a Premium in Straight Equity Sale Involving No Warrants to Advance Rare Disease IND
Investment from largest shareholder strengthens balance sheet, signals insider conviction, and funds Telomir-1's upcoming IND submission for a rare disease indication
MIAMI, FLORIDA / ACCESS Newswire / May 21, 2025 / Telomir Pharmaceuticals, Inc. (NASDAQ:TELO) ("Telomir" or the "Company"), an emerging leader in age-reversal science, today announced it has secured $3 million in equity financing, through a direct investment by The Bayshore Trust, the Company's largest shareholder.
The transaction involved the purchase of 1 million restricted shares of the Company's common stock at $3.00 per share, representing an 18% premium to Telomir's closing share price of $2.54 on the date of execution. The transaction was structured as a straight restricted common stock transaction with no warrants, no discounts, and no convertible features.
This transaction follows a prior $1 million equity investment at $7.00 per share made on December 9, 2024, through The Starwood Trust-an entity affiliated with the Company's largest shareholder - and complements an existing $5 million non-dilutive line of credit from the same affiliated group, which remains undrawn.
"We've now raised $4 million in equity and secured a $5 million credit line - all through affiliated entities on shareholder-friendly terms," said Erez Aminov, Chairman and CEO of Telomir. "These investments included no warrants, no discounts, and no toxic structures. Every financing decision we make is grounded in a long-term view of shareholder value, and this raise reflects that discipline."
From an operational standpoint, our first goal is to submit our IND by year-end and generate early human efficacy data in the most efficient and capital-responsible way. We believe pursuing a rare disease indication gives us a strategic entry point to demonstrate clinical impact and build broader value.
Advancing a Growing Pipeline with Breakthrough Potential
Telomir is advancing two highly innovative drug candidates: Telomir-1, a first-in-class age-reversal molecule targeting the root causes of cellular decline, and Telomir-Ag2, a stabilized Silver(II) compound designed to address the growing threat of drug-resistant infections.
Telomir-1: Reversing Aging, Treating Disease, and Extending Longevity
Telomir-1 is an oral small molecule that addresses five fundamental biological drivers of aging and chronic disease: mitochondrial dysfunction, oxidative stress, calcium imbalance, toxic metal accumulation (iron and copper), and telomere shortening.
In preclinical models, Telomir-1 has demonstrated:
Reversal of the biological clock, improving both lifespan and health span
Improvement of mitochondrial energy production in metabolically stressed cells
Reduction of oxidative stress (ROS), a key contributor to age-related damage
Correction of calcium signaling pathways associated with neurodegeneration and cell death
Protection against metal-induced toxicity from iron and copper
Telomere lengthening and stabilization to support cellular regeneration
Therapeutic potential has been demonstrated across several critical indications:
Progeria: Telomir confirms lifespan restoration and normalization of accelerated aging in a preclinical model of Progeria, a rare genetic disorder causing rapid aging
Type 2 diabetes: Telomir-1 reversed insulin resistance, lowered fasting glucose, and improved glucose homeostasis in zebrafish models
Wilson's disease: Telomir-1 protected cells from copper-induced toxicity, restoring mitochondrial function and reducing oxidative stress
Oncology: In a prostate cancer mice model, Telomir-1 reduced tumor volume by approximately 50%
Chemotherapy support: Co-administration with Paclitaxel prevented mortality in animals otherwise experiencing toxicity
Retinal and neural protection: In vitro studies showed strong protection of human retinal cells from oxidative and metal stress conditions
These results support the advancement of Telomir-1 in multiple rare and high-value indications, including:
Progeria and Werner Syndrome
Wilson's Disease
Type 2 Diabetes
Autism Spectrum Disorder (ASD)
Spasmodic Dysphonia (SD)
Age-related Macular Degeneration (AMD)
Telomir plans to engage with the FDA through the Rare Disease Endpoint Advancement (RDEA) Pilot Program, which supports the development of novel clinical endpoints for underserved conditions. In parallel, the Company is advancing a rare disease indication aligned with Telomir-1's mechanism of action to efficiently generate early human efficacy data and support broader clinical development.
Telomir-Ag2: Stabilized Silver(II) for Drug-Resistant Infections
Telomir-Ag2 is a novel Silver(II) complex stabilized using Telomir's proprietary chelation platform. Silver(II) has historically shown strong antimicrobial potential but has remained clinically impractical due to its instability-until now.
Preclinical studies demonstrate that Telomir-Ag2 is active against:
Escherichia coli
Pseudomonas aeruginosa
Enterococcus faecalis
Staphylococcus aureus
Methicillin- and aminoglycoside-resistant Staphylococcus aureus (MARSA)
Key features include:
Superior antimicrobial performance over Silver(I) in minimum inhibitory concentration (MIC) assays
No sulfa-based compounds, minimizing allergic and cytotoxic risks
Broad potential as a topical product across burn treatment, wound care, and surgical infection prevention
Telomir-Ag2 addresses a growing global market projected to exceed $30 billion across antimicrobial dressings, hospital-acquired infection prevention, and wound care.
"Telomir-Ag2 may be the first stabilized Silver(II) compound viable for medical use," said Dr. Itzchak Angel, Chief Scientific Advisor of the Company. "It's broad-spectrum activity, especially against resistant strains, represents a major advancement in antimicrobial science."
Cautionary Note Regarding Forward-Looking Statements
This press release, statements of Telomir's management or advisors related thereto, and the statements contained in the news story linked in this release contain "forward-looking statements," which are statements other than historical facts made pursuant to the safe harbor provisions of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended. These risks and uncertainties include, but are not limited to, the potential use of the data from our studies, our ability to develop and commercialize Telomir-1 for specific indications, and the safety of Telomir-1.
Any forward-looking statements in this press release are based on Telomir's current expectations, estimates and projections only as of the date of this release. These risks and uncertainties include, but are not limited to, the potential use of the data from our studies, our ability to develop and commercialize Telomir-1 for specific indications and safety of Telomir-1. These and other risks concerning Telomir's programs and operations are described in additional detail in its Annual Report on Form 10-K for the fiscal year ended December 31, 2024, which is on file with the SEC. Telomir explicitly disclaims any obligation to update any forward-looking statements except to the extent required by law.
Contact Information
Helga Moya
[email protected]
(786) 396-6723
SOURCE: Telomir Pharmaceuticals, Inc
View the original press release on ACCESS Newswire
O.Karlsson--AMWN
