
-
 North Korea acknowledges its troops cleared mines for Russia
North Korea acknowledges its troops cleared mines for Russia
-
US unseals warrant for tanker seized off Venezuelan coast

-
 Cambodia says Thailand still bombing hours after Trump truce call
Cambodia says Thailand still bombing hours after Trump truce call
-
Machado urges pressure so Maduro understands 'he has to go'

-
 Leinster stutter before beating Leicester in Champions Cup
Leinster stutter before beating Leicester in Champions Cup
-
World stocks mostly slide, consolidating Fed-fuelled gains

-
 Crypto firm Tether bids for Juventus, is quickly rebuffed
Crypto firm Tether bids for Juventus, is quickly rebuffed
-
Union sink second-placed Leipzig to climb in Bundesliga

-
 US Treasury lifts sanctions on Brazil Supreme Court justice
US Treasury lifts sanctions on Brazil Supreme Court justice
-
UK king shares 'good news' that cancer treatment will be reduced in 2026

-
 Wembanyama expected to return for Spurs in NBA Cup clash with Thunder
Wembanyama expected to return for Spurs in NBA Cup clash with Thunder
-
Five takeaways from Luigi Mangione evidence hearings

-
 UK's king shares 'good news' that cancer treatment will be reduced in 2026
UK's king shares 'good news' that cancer treatment will be reduced in 2026
-
Steelers' Watt undergoes surgery to repair collapsed lung

-
 Iran detains Nobel-prize winner in 'brutal' arrest
Iran detains Nobel-prize winner in 'brutal' arrest
-
NBA Cup goes from 'outside the box' idea to smash hit

-
 UK health service battles 'super flu' outbreak
UK health service battles 'super flu' outbreak
-
Can Venezuela survive US targeting its oil tankers?

-
 Democrats release new cache of Epstein photos
Democrats release new cache of Epstein photos
-
Colombia's ELN guerrillas place communities in lockdown citing Trump 'intervention' threats

-
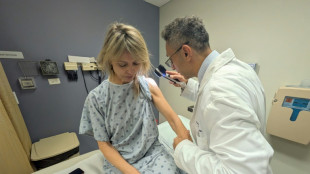 'Don't use them': Tanning beds triple skin cancer risk, study finds
'Don't use them': Tanning beds triple skin cancer risk, study finds
-
Nancy aims to restore Celtic faith with Scottish League Cup final win

-
 Argentina fly-half Albornoz signs for Toulon until 2030
Argentina fly-half Albornoz signs for Toulon until 2030
-
Trump says Thailand, Cambodia have agreed to stop border clashes

-
 Salah in Liverpool squad for Brighton after Slot talks - reports
Salah in Liverpool squad for Brighton after Slot talks - reports
-
Marseille coach tips Greenwood as 'potential Ballon d'Or'

-
 Draw marks 'starting gun' toward 2026 World Cup, Vancouver says
Draw marks 'starting gun' toward 2026 World Cup, Vancouver says
-
Thai PM says asked Trump to press Cambodia on border truce

-
 Salah admired from afar in his Egypt home village as club tensions swirl
Salah admired from afar in his Egypt home village as club tensions swirl
-
World stocks retrench, consolidating Fed-fuelled gains

-
 Brazil left calls protests over bid to cut Bolsonaro jail time
Brazil left calls protests over bid to cut Bolsonaro jail time
-
Trump attack on Europe migration 'disaster' masks toughening policies

-
 US plan sees Ukraine joining EU in 2027, official tells AFP
US plan sees Ukraine joining EU in 2027, official tells AFP
-
'Chilling effect': Israel reforms raise press freedom fears

-
 Iran frees child bride sentenced to death over husband's killing: activists
Iran frees child bride sentenced to death over husband's killing: activists
-
No doubting Man City boss Guardiola's passion says Toure

-
 Youthful La Rochelle name teen captain for Champions Cup match in South Africa
Youthful La Rochelle name teen captain for Champions Cup match in South Africa
-
World stocks consolidate Fed-fuelled gains

-
 British 'Aga saga' author Joanna Trollope dies aged 82
British 'Aga saga' author Joanna Trollope dies aged 82
-
Man Utd sweat on Africa Cup of Nations trio

-
 EU agrees three-euro small parcel tax to tackle China flood
EU agrees three-euro small parcel tax to tackle China flood
-
Taylor Swift breaks down in Eras documentary over Southport attack

-
 Maresca 'relaxed' about Chelsea's rough patch
Maresca 'relaxed' about Chelsea's rough patch
-
France updates net-zero plan, with fossil fuel phaseout

-
 Nowhere to pray as logs choke flood-hit Indonesian mosque
Nowhere to pray as logs choke flood-hit Indonesian mosque
-
In Pakistan, 'Eternal Love' has no place on YouTube

-
 England bowling great Anderson named as Lancashire captain
England bowling great Anderson named as Lancashire captain
-
UK's King Charles to give personal TV message about cancer 'journey'

-
 Fit-again Jesus can be Arsenal's number one striker, says Arteta
Fit-again Jesus can be Arsenal's number one striker, says Arteta
-
Spain's ruling Socialists face sex scandal fallout among women voters


MIRA Pharmaceuticals to Participate in BIO 2025 in Boston and Highlights Ongoing Progress Across Clinical Program
The company will engage in BIO One-on-One Partnering™ meetings as it advances Phase 1 for Ketamir-2, prepares Phase IIa study in neuropathic pain, and finalizes filings for SKNY acquisition.
MIAMI, FL / ACCESS Newswire / May 28, 2025 / MIRA Pharmaceuticals, Inc. (Nasdaq:MIRA) ("MIRA" or the "Company"), a clinical-stage pharmaceutical company developing novel therapeutics for neurologic, neuropsychiatric, and metabolic disorders, today announced that it will participate in the BIO International Convention 2025, taking place in Boston, MA from June 16-19, 2025. The Company has a full schedule of BIO One-on-One Partnering™ meetings planned as it explores potential licensing, strategic partnerships, and M&A opportunities.
The Company's lead candidate, Ketamir-2, a next-generation oral ketamine analog, is currently undergoing a Phase 1 clinical trial. With the second dosing cohort completed, the Company is now preparing to initiate the third cohort. Building on this momentum, MIRA anticipates initiating a Phase IIa study in neuropathic pain before the end of the year, advancing the development of what the Company believes could be a safe, effective non-opioid alternative for chronic pain management.
In addition, MIRA is advancing a series of preclinical studies with Ketamir-2, including models evaluating its potential in PTSD, as well as a topical formulation aimed at treating localized inflammatory pain. The Company is also finalizing regulatory filings related to its acquisition of SKNY Pharmaceuticals, Inc. ("SKNY"), with submission to the U.S. Securities and Exchange Commission (SEC) expected in the coming weeks. SKNY-1, SKNY's primary pharmaceutical candidate, is being developed as an oral therapeutic targeting smoking cessation and obesity, with activity at CB1, CB2, and MAO-B receptors.
"Our pipeline is advancing on all fronts, and we are focused on turning this scientific momentum into long-term value for patients and shareholders," said Erez Aminov, Chief Executive Officer of MIRA. "As we move closer to initiating Phase IIa and completing the SKNY transaction, we're actively exploring strategic opportunities to accelerate growth, including licensing and partnerships-especially in areas like chronic pain where non-opioid alternatives like Ketamir-2 are urgently needed."
Dr. Angel, Chief Scientific Advisor at MIRA, added:
"We believe Ketamir-2 is paving the way for a new class of non-opioid therapies. The science is compelling, and the progress we have made is truly exciting. I look forward to sharing the depth of our work and the promising data we've generated with potential partners and investors."
Cautionary Note Regarding Forward-Looking Statements
This press release and the statements of MIRA's management related thereto contain "forward-looking statements," which are statements other than historical facts made pursuant to the safe harbor provisions of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended. These statements may be identified by words such as "aims," "anticipates," "believes," "could," "estimates," "expects," "forecasts," "goal," "intends," "may," "plans," "possible," "potential," "seeks," "will," and variations of these words or similar expressions that are intended to identify forward-looking statements. Any statements in this press release that are not historical facts may be deemed forward-looking. Any forward-looking statements in this press release are based on MIRA's current expectations, estimates, and projections only as of the date of this release and are subject to a number of risks and uncertainties (many of which are beyond MIRA's control) that could cause actual results to differ materially and adversely from those set forth in or implied by such forward-looking statements, including related to MIRA's potential merger with SKNY Pharmaceuticals, Inc. These and other risks concerning MIRA's programs and operations are described in additional detail in the Annual Report on Form 10-K for the year ended December 31, 2024, and other SEC filings, which are on file with the SEC at www.sec.gov and MIRA's website at https://www.mirapharmaceuticals.com/investors/sec-filings. MIRA explicitly disclaims any obligation to update any forward-looking statements except to the extent required by law.
Contact Information
Helga Moya
[email protected]
(786) 432-9792
SOURCE: MIRA Pharmaceuticals
View the original press release on ACCESS Newswire
A.Mahlangu--AMWN