
-
 Second woman accuses French senator of drugging her
Second woman accuses French senator of drugging her
-
Russian government, central bank spar over economic downturn

-
 Thai PM meets army commander in attempt to defuse political crisis
Thai PM meets army commander in attempt to defuse political crisis
-
More microplastics in glass bottles than plastic: study

-
 Top Iran, EU diplomats to hold nuclear talks
Top Iran, EU diplomats to hold nuclear talks
-
Armenia PM arrives in Turkey for 'historic' visit

-
 Salah among nominees for PFA Player of the Year award
Salah among nominees for PFA Player of the Year award
-
EU bars Chinese firms from major state medical equipment contracts

-
 Three-time world champion figure skater Sakamoto to retire
Three-time world champion figure skater Sakamoto to retire
-
Crude sinks as Trump delays decision on Iran strike

-
 Two dead in Mexico as Hurricane Erick moves on from Mexican coast
Two dead in Mexico as Hurricane Erick moves on from Mexican coast
-
US appeals court allows Trump control of National Guard in LA

-
 Monsters and memes: Labubu dolls ride China soft-power wave
Monsters and memes: Labubu dolls ride China soft-power wave
-
Chad hopes 'green charcoal' can save vanishing forests

-
 'Turkish salmon': the Black Sea's new rose-coloured gold
'Turkish salmon': the Black Sea's new rose-coloured gold
-
Rays pitcher Bigge hospitalized after being struck by foul ball

-
 PSG stunned by Botafogo after Messi lights up Club World Cup
PSG stunned by Botafogo after Messi lights up Club World Cup
-
Thunder ready to play for all the marbles - Gilgeous-Alexander

-
 Europe's lithium quest hampered by China and lack of cash
Europe's lithium quest hampered by China and lack of cash
-
Japan-US-Philippines hold coast guard drills with eye on China

-
 Richards strike gives USA spot in Gold Cup quarters
Richards strike gives USA spot in Gold Cup quarters
-
Pacers thrash Thunder to stay alive in NBA Finals
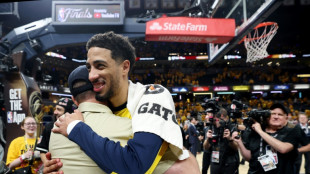
-
 Cheap alms bowls imports hit Sri Lanka makers, monks
Cheap alms bowls imports hit Sri Lanka makers, monks
-
Pacers demolish Thunder to stay alive in NBA Finals

-
 PSG stunned by Botafogo in Club World Cup upset
PSG stunned by Botafogo in Club World Cup upset
-
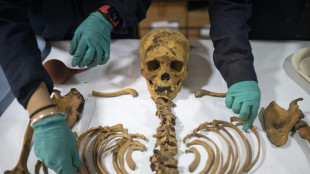
Peru gas workers find thousand-year-old mummy
-
 UK MPs to hold crunch vote on assisted dying
UK MPs to hold crunch vote on assisted dying
-
Australian trial says tech for social media teen ban can work

-
 Thai PM to meet army commander to defuse political crisis
Thai PM to meet army commander to defuse political crisis
-
Rice prices double in Japan as inflation accelerates

-
 Summoning golden Olympic memories, Paris parties like it's 2024
Summoning golden Olympic memories, Paris parties like it's 2024
-
Peru's Maido named world's top restaurant on 50 Best list

-
 US singer Chris Brown in London court on assault case
US singer Chris Brown in London court on assault case
-
Thailand credits prey releases for 'extraordinary' tiger recovery

-
 Can NATO keep Trump on-message about Russia threat?
Can NATO keep Trump on-message about Russia threat?
-
Australia drop struggling Labuschagne for first West Indies Test

-
 European, Iranian diplomats to meet as US mulls joining Israel campaign
European, Iranian diplomats to meet as US mulls joining Israel campaign
-
Paris makes clean water bet for River Seine bathers

-
 Jeeno Thitikul edges clear as heat takes toll at Women's PGA
Jeeno Thitikul edges clear as heat takes toll at Women's PGA
-
Critic of Nicaragua's Ortega shot dead in exile in Costa Rica

-
 Barrios double gets Atletico back on track
Barrios double gets Atletico back on track
-
Tencent Cloud Empowers Ryde to Elevate In-App Communication in Southeast Asia
-
 Alpha Growth PLC - Providence Life Granted Isle of Man Branch License
Alpha Growth PLC - Providence Life Granted Isle of Man Branch License
-
Relief Therapeutics Receives FDA Response to QIDP Request for RLF-TD011

-
 World No. 1 Scheffler shares lead at PGA Travelers Championship
World No. 1 Scheffler shares lead at PGA Travelers Championship
-
Messi's 'winning spirit' surprising: Inter Miami's Mascherano

-
 US immigration agents barred from LA Dodgers' stadium: team
US immigration agents barred from LA Dodgers' stadium: team
-
SpaceX Starship explodes on Texas launch pad
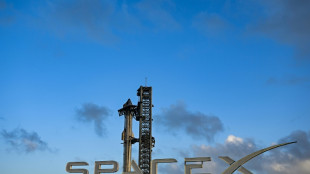
-
 Messi strikes as Inter Miami stun Porto at Club World Cup
Messi strikes as Inter Miami stun Porto at Club World Cup
-
US immigration agents barred from LA baseball stadium: team


Relief Therapeutics Receives FDA Response to QIDP Request for RLF-TD011
GENEVA, SWITZERLAND / ACCESS Newswire / June 20, 2025 / RELIEF THERAPEUTICS Holding SA (SIX:RLF)(OTCQB:RLFTF)(OTCQB:RLFTY ) (Relief or the Company), a biopharmaceutical company committed to delivering innovative treatment options for select specialty, unmet and rare diseases, today announced that its initial request for Qualified Infectious Disease Product (QIDP) designation for RLF-TD011 for the treatment of epidermolysis bullosa (EB) was not granted by the U.S. Food and Drug Administration (FDA). The FDA determined that the investigational therapy does not currently meet the criteria for QIDP designation.

This outcome does not impact the Company's development and regulatory strategy for RLF-TD011. The program continues to benefit from both Orphan Drug and Rare Pediatric Disease designations, which provide important regulatory and commercial incentives, including the potential for seven years of market exclusivity in the United States upon approval. RLF-TD011 is further supported by the Company's intellectual property portfolio, including a recently granted European patent providing protection through 2040.
The Company also reported that it held a constructive pre-Investigational New Drug (pre-IND) meeting with the FDA. Discussions focused on the remaining clinical and regulatory steps required to advance RLF‑TD011 into later-stage development. Relief expects to complete the pre-IND meeting process upon receipt of the FDA's written responses in the coming weeks.
ABOUT EPIDERMOLYSIS BULLOSA
Epidermolysis bullosa (EB) is a group of rare, inherited connective tissue disorders characterized by extreme skin fragility, leading to blistering and wounds from minor friction or injury. In severe cases, blisters can develop into chronic wounds or form in internal organs such as the mouth or esophagus, leading to painful wounds, recurrent infections, and a deeply impacted quality of life. EB is classified into several major inherited subtypes, each defined by the depth of blister formation within the skin's layers: epidermolysis bullosa simplex (EBS), dystrophic epidermolysis bullosa (DEB), junctional epidermolysis bullosa (JEB) and Kindler syndrome (KS). Treatment is intensive and includes wound care, infection prevention, and pain management. Approximately 500,000 individuals worldwide are affected by EB.
ABOUT RLF-TD011
RLF-TD011 is a highly pure, stabilized hypochlorous acid solution developed using Relief's proprietary TEHCLO™ technology. With strong antimicrobial properties, RLF-TD011 is a sprayable, self-administered solution for targeted wound application while avoiding skin contact and cross-contamination. Relief's acid-oxidizing solution has previously demonstrated efficacy in accelerating wound closure and reducing infections in certain clinical trials on non-EB wounds. In an investigator-initiated trial (NCT05533866), RLF‑TD011 has also shown promising results in infection control and wound healing in EB patients with the most severe forms of the disease. RLF-TD011 aims to address unmet needs in EB care by efficiently controlling infection and inflammation while reducing antibiotic use and easing the intensive, time-consuming wound care routine required by current treatments. The U.S. Food and Drug Administration has granted RLF-TD011 both Orphan Drug and Rare Pediatric Disease designations for EB.
ABOUT RELIEF
Relief is a commercial-stage biopharmaceutical company dedicated to advancing treatment paradigms and improving the lives of patients with rare and debilitating diseases. With core expertise in drug delivery systems and drug repurposing, Relief's clinical pipeline includes innovative treatments designed to address critical unmet medical needs in rare dermatological, metabolic and respiratory conditions. The Company has also successfully brought several approved products to market through licensing and distribution partnerships. Headquartered in Geneva, Relief is listed on the SIX Swiss Exchange under the symbol RLF and quoted in the U.S. on OTCQB under the symbols RLFTF and RLFTY. For more information, visit www.relieftherapeutics.com
CONTACT
RELIEF THERAPEUTICS Holding SA
Jeremy Meinen
Chief Financial Officer
[email protected]
DISCLAIMER
This press release contains forward-looking statements, which may be identified by words such as "believe," "assume," "expect," "intend," "may," "could," "will," or similar expressions. These statements are based on current plans and assumptions and are subject to risks and uncertainties that could cause actual results, financial condition, performance, or achievements to differ materially from those expressed or implied. Such factors include, but are not limited to, changes in economic conditions, market developments, regulatory changes, competitive dynamics, and other risks or changes in circumstances. This communication is provided as of the date hereof, and Relief undertakes no obligation to update any forward-looking statements contained herein as a result of new information, future events or otherwise.
SOURCE: Relief Therapeutics Holding SA
View the original press release on ACCESS Newswire
L.Mason--AMWN