
-
 UK marks London 7/7 attacks as king hails 'spirit of unity'
UK marks London 7/7 attacks as king hails 'spirit of unity'
-
Apple appeals 500-mn-euro EU fine

-
 Crowds celebrate Nepal ex-king's birthday in show of support
Crowds celebrate Nepal ex-king's birthday in show of support
-
Bali flights nixed after huge Indonesia volcano eruption

-
 Hamas, Israel resume talks as Netanyahu set to meet Trump
Hamas, Israel resume talks as Netanyahu set to meet Trump
-
Hong Kong fans queue for opening of Cristiano Ronaldo exhibition

-
 Itoje back as Lions take no chances against ACT Brumbies
Itoje back as Lions take no chances against ACT Brumbies
-
Stock markets struggle as Trump's tariff deadline looms

-
 Nearly 450,000 Afghans left Iran since June 1: IOM
Nearly 450,000 Afghans left Iran since June 1: IOM
-
North Korea bars Western influencers from trade fair tour

-
 Typhoon Danas kills two, injures hundreds in Taiwan
Typhoon Danas kills two, injures hundreds in Taiwan
-
Dutch coastal village turns to tech to find lost fishermen

-
 Boxer Chavez's appeal against arrest if deported from US rejected: Mexico prosecutor
Boxer Chavez's appeal against arrest if deported from US rejected: Mexico prosecutor
-
India captain Gill hailed back home after 'brilliant' Test win

-
 The making of Australia's mushroom murders
The making of Australia's mushroom murders
-
Indonesia volcano spews 18-kilometre ash tower

-
 Youthful Chelsea ready for Thiago Silva reunion at Club World Cup
Youthful Chelsea ready for Thiago Silva reunion at Club World Cup
-
Australian inquiry cites racism in Indigenous shooting

-
 Djokovic wary despite Wimbledon form, dominant Sinner faces Dimitrov
Djokovic wary despite Wimbledon form, dominant Sinner faces Dimitrov
-
Australian woman found guilty of triple murder with toxic mushrooms

-
 Indonesia volcano spews 18-kilometre ash tower: agency
Indonesia volcano spews 18-kilometre ash tower: agency
-
Trump says to send first tariff letters on Monday

-
 The strange case of Evgeniya Mayboroda, Russia's rebel retiree
The strange case of Evgeniya Mayboroda, Russia's rebel retiree
-
Asian markets drop as Trump's tariff deadline looms

-
 Under-strength Brumbies eye 'big opportunity' against Lions
Under-strength Brumbies eye 'big opportunity' against Lions
-
Macron to rekindle relationship with Francophile King Charles on UK visit

-
 Trump hosts Netanyahu, hopes for Israel-Hamas deal 'this week'
Trump hosts Netanyahu, hopes for Israel-Hamas deal 'this week'
-
Pressed to confess: Japan accused of 'hostage justice'

-
 Demna to bow out at Balenciaga in Paris Haute Couture Week
Demna to bow out at Balenciaga in Paris Haute Couture Week
-
Host of internationals in Australia-New Zealand squad to face Lions
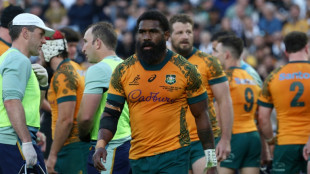
-
 Egyptian conservators give King Tut's treasures new glow
Egyptian conservators give King Tut's treasures new glow
-
Mexico defeat USA 2-1 to retain CONCACAF Gold Cup

-
 Visa's 24/7 war room takes on global cybercriminals
Visa's 24/7 war room takes on global cybercriminals
-
BRICS nations slam Trump tariffs, condemn strikes on Iran

-
 Fineqia's Yield-Bearing Bitcoin ETP Garners $13.9 Mln; Total Co. AUM Reaches $36 Mln
Fineqia's Yield-Bearing Bitcoin ETP Garners $13.9 Mln; Total Co. AUM Reaches $36 Mln
-
BioNxt's Sublingual Cladribine Program for MS Ready for Next Phase

-
 Guardian Metal Resources PLC Announces Pilot North Tungsten Project Acquired via Staking
Guardian Metal Resources PLC Announces Pilot North Tungsten Project Acquired via Staking
-
MLB Nationals fire manager Martinez, GM Rizzo after loss

-
 US tariffs to kick in Aug 1, barring trade deals
US tariffs to kick in Aug 1, barring trade deals
-
Trump slams former ally Musk's political party as 'ridiculous'

-
 Three things we learned from the second England-India Test
Three things we learned from the second England-India Test
-
Norway reach Euro 2025 quarter-finals as Swiss down eliminated Iceland
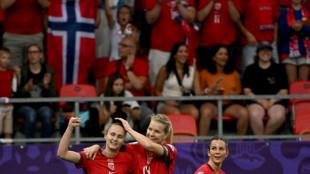
-
 Alcaraz vows to avoid Murray after defeat on golf course
Alcaraz vows to avoid Murray after defeat on golf course
-
Alcaraz finds magic touch at Wimbledon as Sabalenka storms into quarter-finals

-
 Run-hungry Gill glad to 'lead by example' as India level England series
Run-hungry Gill glad to 'lead by example' as India level England series
-
Rockets confirm arrival of Durant in unprecedented NBA seven-team trade

-
 Alcaraz survives Rublev test to stay on course for Wimbledon hat-trick
Alcaraz survives Rublev test to stay on course for Wimbledon hat-trick
-
New Zealand's Dixon wins seventh IndyCar Mid-Ohio title

-
 US tariffs to kick in Aug 1, barring trade deals: Bessent
US tariffs to kick in Aug 1, barring trade deals: Bessent
-
England consider Archer and Atkinson recall after heavy India defeat


BioNxt's Sublingual Cladribine Program for MS Ready for Next Phase
VANCOUVER, BC / ACCESS Newswire / July 7, 2025 / BioNxt Solutions Inc. ("BioNxt" or the "Company") (CSE:BNXT)(OTC:XPHYF)(FSE:4XT), a bioscience innovator specializing in advanced drug delivery systems, is pleased to announce the receipt of the active pharmaceutical ingredient (API) for its lead product candidate, BNT23001. This proprietary sublingual thin-film formulation of cladribine is being developed for the treatment of multiple sclerosis (MS).
The delivery of the cladribine API enables the commencement of clinical batch manufacturing in collaboration with BioNxt's European contract research and development partner, Gen-Plus GmbH & Co KG, based in Munich, Germany. This milestone supports the company's plan to initiate a human bioequivalence study in the second half of 2025.
BNT23001 is designed to offer a patient-friendly alternative to traditional oral cladribine tablets, such as Mavenclad®, by providing a rapidly dissolving sublingual film. This delivery method aims to enhance patient compliance, particularly for individuals with MS who experience dysphagia, a common symptom affecting the ability to swallow.
Preclinical studies have demonstrated that BNT23001 achieves high absorption rates and bioequivalence to existing oral therapies, with no adverse toxic effects observed. These findings support the progression to human bioequivalence studies.
BioNxt has also made significant strides in securing intellectual property rights for BNT23001. The company has initiated patent nationalization processes in key jurisdictions, including Europe, the United States, and Canada, with patent grants anticipated by mid-2025.
"The receipt of the cladribine API is a pivotal step forward in our mission to develop innovative, patient-centric therapies for MS," said Hugh Rogers, CEO of BioNxt Solutions. "We are committed to advancing BNT23001 through clinical development and bringing this novel treatment option to patients worldwide."
About BioNxt Solutions Inc.
BioNxt Solutions Inc. is a bioscience innovator focused on next‐generation drug delivery technologies, diagnostic screening systems, and active pharmaceutical ingredient development. The Company's proprietary platforms-Sublingual (Thin‐Film), Transdermal (Skin Patch), and Oral (Enteric‐Coated Tablets)-target key therapeutic areas, including autoimmune diseases, neurological disorders, and longevity.
With research and development operations in North America and Europe, BioNxt is advancing regulatory approvals and commercialization efforts, primarily focused on European markets. BioNxt is committed to improving healthcare by delivering precise, patient‐centric solutions that enhance treatment outcomes worldwide.
BioNxt is listed on the Canadian Securities Exchange: BNXT, OTC Markets: BNXTF and trades in Germany under WKN: A3D1K3. To learn more about BioNxt, please visit www.bionxt.com.
Investor Relations & Media Contact
Hugh Rogers, Co‐Founder, CEO and Director
Email: [email protected]
Phone: +1 778.598.2698
Web: www.bionxt.com
LinkedIn: https://www.linkedin.com/company/bionxt‐solutions
Instagram: https://www.instagram.com/bionxt
Cautionary Statement Regarding "Forward‐Looking" Information
This news release includes certain statements that may be deemed "forward-looking statements". All statements in this release, other than statements of historical facts, that address events or developments that the Company expects to occur, are forward-looking statements. Forward-looking statements are statements that are not historical facts and are generally, but not always, identified by the words "expects", "plans", "anticipates", "believes", "intends", "estimates", "projects", "potential" and similar expressions, or that events or conditions "will", "would", "may", "could" or "should" occur. Forward-looking information in this news release includes the anticipated filing date of the Annual Filings. Although the Company believes the expectations expressed in such forward-looking statements are based on reasonable assumptions, such statements are not guarantees of future performance and actual results may differ materially from those in the forward-looking statements. Factors that could cause the actual results to differ materially from those in forward-looking statements include regulatory actions, market prices, and continued availability of capital and financing, and general economic, market or business conditions. Investors are cautioned that any such statements are not guarantees of future performance and actual results or developments may differ materially from those projected in the forward-looking statements. Forward-looking statements are based on the beliefs, estimates and opinions of the Company's management on the date the statements are made. Except as required by applicable securities laws, the Company undertakes no obligation to update these forward-looking statements in the event that management's beliefs, estimates or opinions, or other factors, should change.
SOURCE: BioNxt Solutions Inc.
View the original press release on ACCESS Newswire
X.Karnes--AMWN