
-
 Right wing urges boycott of iconic Brazilian flip-flops
Right wing urges boycott of iconic Brazilian flip-flops
-
From misfits to MAGA: Nicki Minaj's political whiplash

-
 Foster grabs South Africa winner against Angola in AFCON
Foster grabs South Africa winner against Angola in AFCON
-
Russia pledges 'full support' for Venezuela against US 'hostilities'

-
 Spotify says piracy activists hacked its music catalogue
Spotify says piracy activists hacked its music catalogue
-
Winter Olympics organisers resolve snow problem at ski site

-
 Fuming Denmark summons US ambassador over Greenland envoy
Fuming Denmark summons US ambassador over Greenland envoy
-
UK's street artist Banksy unveils latest mural in London

-
 Rugby players lose order challenge in brain injury claim
Rugby players lose order challenge in brain injury claim
-
UK singer Chris Rea dies at 74, days before Christmas

-
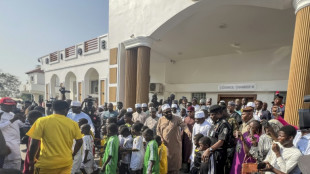 Last of kidnapped Nigerian pupils handed over, government says
Last of kidnapped Nigerian pupils handed over, government says
-
Zambia strike late to hold Mali in AFCON opener

-
 Outcry follows CBS pulling program on prison key to Trump deportations
Outcry follows CBS pulling program on prison key to Trump deportations
-
Sri Lanka cyclone caused $4.1 bn damage: World Bank

-
 Billionaire Ellison offers personal guarantee for son's bid for Warner Bros
Billionaire Ellison offers personal guarantee for son's bid for Warner Bros
-
Tech stocks lead Wall Street higher, gold hits fresh record

-
 Telefonica to shed around 5,500 jobs in Spain
Telefonica to shed around 5,500 jobs in Spain
-
McCullum wants to stay as England coach despite Ashes drubbing

-
 EU slams China dairy duties as 'unjustified'
EU slams China dairy duties as 'unjustified'
-
Italy fines Apple nearly 100 mn euros over app privacy feature

-
 America's Cup switches to two-year cycle
America's Cup switches to two-year cycle
-
Jesus could start for Arsenal in League Cup, says Arteta

-
 EU to probe Czech aid for two nuclear units
EU to probe Czech aid for two nuclear units
-
Strauss says sacking Stokes and McCullum will not solve England's Ashes woes

-
 Noel takes narrow lead after Alta Badia slalom first run
Noel takes narrow lead after Alta Badia slalom first run
-
Stocks diverge as rate hopes rise, AI fears ease

-
 Man City players face Christmas weigh-in as Guardiola issues 'fatty' warning
Man City players face Christmas weigh-in as Guardiola issues 'fatty' warning
-
German Christmas markets hit by flood of fake news

-
 Liverpool fear Isak has broken leg: reports
Liverpool fear Isak has broken leg: reports
-
West Indies captain says he 'let the team down' in New Zealand Tests

-
 Thailand says Cambodia agrees to border talks after ASEAN meet
Thailand says Cambodia agrees to border talks after ASEAN meet
-
Alleged Bondi shooters conducted 'tactical' training in countryside, Australian police say

-
 Swiss court to hear landmark climate case against cement giant
Swiss court to hear landmark climate case against cement giant
-
Knicks' Brunson scores 47, Bulls edge Hawks epic

-
 Global nuclear arms control under pressure in 2026
Global nuclear arms control under pressure in 2026
-
Asian markets rally with Wall St as rate hopes rise, AI fears ease

-
 Jailed Malaysian ex-PM Najib loses bid for house arrest
Jailed Malaysian ex-PM Najib loses bid for house arrest
-
Banned film exposes Hong Kong's censorship trend, director says

-
 Duffy, Patel force West Indies collapse as NZ close in on Test series win
Duffy, Patel force West Indies collapse as NZ close in on Test series win
-
Australian state pushes tough gun laws, 'terror symbols' ban after shooting

-
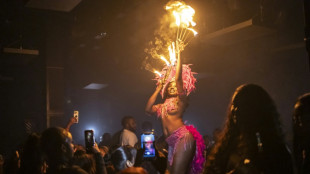 A night out on the town during Nigeria's 'Detty December'
A night out on the town during Nigeria's 'Detty December'
-
US in 'pursuit' of third oil tanker in Caribbean: official

-
 CO2 soon to be buried under North Sea oil platform
CO2 soon to be buried under North Sea oil platform
-
Steelers edge Lions as Bears, 49ers reach playoffs

-
 India's Bollywood counts costs as star fees squeeze profits
India's Bollywood counts costs as star fees squeeze profits
-
McCullum admits errors in Ashes preparations as England look to salvage pride

-
 Pets, pedis and peppermints: When the diva is a donkey
Pets, pedis and peppermints: When the diva is a donkey
-
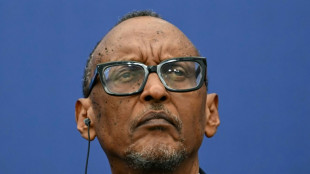
'A den of bandits': Rwanda closes thousands of evangelical churches
-
 Southeast Asia bloc meets to press Thailand, Cambodia on truce
Southeast Asia bloc meets to press Thailand, Cambodia on truce
-
As US battles China on AI, some companies choose Chinese

| SCS | 0.12% | 16.14 | $ | |
| RYCEF | -0.71% | 15.5 | $ | |
| RBGPF | 0.96% | 81 | $ | |
| VOD | 0.16% | 12.86 | $ | |
| CMSD | -0.22% | 23.2 | $ | |
| CMSC | 0.34% | 23.25 | $ | |
| NGG | 0.16% | 76.235 | $ | |
| RELX | 0.57% | 40.963 | $ | |
| BCE | -1.22% | 22.565 | $ | |
| RIO | 2.29% | 80.155 | $ | |
| BTI | 0.27% | 56.6 | $ | |
| GSK | -0.09% | 48.565 | $ | |
| AZN | 0.11% | 91.465 | $ | |
| JRI | -0.08% | 13.369 | $ | |
| BCC | -0.88% | 74.115 | $ | |
| BP | 0.83% | 34.225 | $ |

Aptevo Highlights APVO442, a CD3-Directed Preclinical Candidate for Prostate Cancer
Builds on the targeted immune activation model validated by mipletamig in AML, adapted for prostate cancer.
Engineered for precision T-cell activation in prostate cancer; part of Aptevo's growing CRIS-7-derived CD3 portfolio targeting both hematologic and solid tumors
SEATTLE, WA / ACCESS Newswire / August 13, 2025 / Aptevo Therapeutics Inc. (Nasdaq:APVO), a clinical-stage biotechnology company focused on developing novel immune-oncology therapeutics based on its proprietary ADAPTIR® and ADAPTIR-FLEX® platform technologies, today reinforced the strategic importance of its preclinical asset APVO442-an investigational CD3-engaging bispecific antibody designed to treat prostate cancer. APVO442 is built on Aptevo's next-generation ADAPTIR-FLEX platform and is engineered to selectively activate T cells within the PSMA-expressing tumor microenvironment, offering potential for precision targeting and reduced systemic toxicity.
APVO442 is built on the same CRIS-7-derived anti-CD3 binding domain as clinical candidate mipletamig but is specifically engineered for solid tumors, with lower binding affinity and a monovalent format that reduce the risk of immune activation outside the tumor. This design helps ensure that T cells are activated only within the tumor microenvironment, improving safety while maintaining anti-tumor potency. Preclinical data show that APVO442 effectively localizes to PSMA-expressing prostate tumors, triggering a targeted immune response while potentially sparing healthy tissue. The same solid tumor-optimized architecture has also been applied to newly added pipeline candidate APVO455.
"With mipletamig performing as designed in the clinic and the addition to the pipeline of APVO455 for multiple solid tumors, we're confident in the strength of our CD3-based bispecific approach," said Marvin White, President and CEO of Aptevo. "APVO442 is engineered for the same balance-potent immune activation precisely where it's needed - in the tumor. It represents yet another high-conviction opportunity to expand into solid tumors, a market where the need is large and growing."
Prostate cancer is the second most common cancer in men, with over 300,000 new cases annually* in the U.S. The global treatment market-currently valued at $14 billion-is projected to reach $24 billion within the next decade**. APVO442 is differentiated in this space as a tumor-specific immunotherapy designed for combination potential and scalable manufacturing. (*American Cancer Society, **Global Data)
APVO442 is currently in preclinical studies, and supports the Company's goal of expanding its clinical pipeline and driving long-term value through modular, platform-based innovation. The Company continues to leverage insights from its CD3 bispecific programs to accelerate future development and partnership opportunities.
About Aptevo Therapeutics
Aptevo Therapeutics Inc. (Nasdaq:APVO) is a clinical-stage biotechnology company focused on developing novel bispecific immunotherapies for the treatment of cancer. The Company has two clinical candidates. Mipletamig is currently being evaluated in RAINIER, a two-part Phase 1b/2 trial for the treatment of frontline acute myeloid leukemia in combination with standard-of-care venetoclax + azacitidine. Mipletamig has received orphan drug designation ("orphan status") for AML according to the Orphan Drug Act. ALG.APV-527, a bispecific conditional 4-1BB agonist, only active upon simultaneous binding to 4-1BB and 5T4, is being co-developed with Alligator Bioscience and successfully completed a Phase 1 clinical trial for the treatment of multiple solid tumor types likely to express 5T4. The Company has four pre-clinical candidates with different mechanisms of action designed to target a range of solid tumors. All pipeline candidates were created from two proprietary platforms, ADAPTIR®and ADAPTIR-FLEX®. The Aptevo mission is to improve treatment outcomes and transform the lives of cancer patients. For more information, please visit www.aptevotherapeutics.com.
Safe Harbor Statement
This press release includes forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. All statements, other than statements of historical fact, including, without limitation, Aptevo's expectations about the activity, efficacy, safety, tolerability and durability of its therapeutic candidates and potential use of any such candidates, including in combination with other drugs, as therapeutics for treatment of disease, its expectations regarding the effectiveness of its ADAPTIR and ADAPTIR-FLEX platforms, whether pre-clinical studies of APVO442 will show the desired anti-tumor efficacy, mechanism of action and safety profile and whether APVO442 will function with new mechanisms of action compared to our previous candidates and synergistically induce a biological response, whether APVO442 will demonstrate the ability to fight prostate cancer, statements related to the progress of Aptevo's clinical programs, including statements related to anticipated clinical and regulatory milestones, whether further study of mipletamig in a Phase 1b dose optimization trial focusing on multiple doses of mipletamig in combination with venetoclax + azacitidine on a targeted patient population will continue to show clinical benefit, whether Aptevo's final trial results will vary from its earlier assessment, the possibility and timing of interim data readouts for ALG.APV-527, statements related to Aptevo's ability to generate stockholder value, whether Aptevo will continue to have momentum in its business in the future, and any other statements containing the words "may," "continue to," "believes," "knows," "expects," "optimism," "potential," "designed," "promising," "plans," "will" and similar expressions are intended to identify forward-looking statements. These forward-looking statements are based on Aptevo's current intentions, beliefs, and expectations regarding future events. Aptevo cannot guarantee that any forward-looking statement will be accurate. Investors should realize that if underlying assumptions prove inaccurate or unknown risks or uncertainties materialize, actual results could differ materially from Aptevo's expectations. Investors are, therefore, cautioned not to place undue reliance on any forward-looking statement.
There are several important factors that could cause Aptevo's actual results to differ materially from those indicated by such forward-looking statements, including a deterioration in Aptevo's business or prospects; further assessment of preliminary or interim data or different results from later clinical trials; adverse events and unanticipated problems, adverse developments in clinical development, including unexpected safety issues observed during a clinical trial; and changes in regulatory, social, macroeconomic and political conditions. For instance, actual results may differ materially from those indicated by such forward-looking statements as a result of various important factors, including the uncertainties inherent in the results of preliminary or interim data and preclinical studies being predictive of the results of later-stage clinical trials, initiation, enrollment and maintenance of patients, and the completion of clinical trials, the availability and timing of data from ongoing clinical trials, the trial design includes combination therapies that may make it difficult to accurately ascertain the benefits of mipletamig, expectations for the timing and steps required in the regulatory review process, expectations for regulatory approvals, the impact of competitive products, our ability to enter into agreements with strategic partners or raise funds on acceptable terms or at all and other matters that could affect the availability or commercial potential of Aptevo's product candidates, business or economic disruptions due to catastrophes or other events, including natural disasters or public health crises, geopolitical risks, including the current wars between Russia and Ukraine, Israel and Hamas, Israel and Iran, and any other military event that could evolve out of any of the current conflicts and macroeconomic conditions such as economic uncertainty, imposition of tariffs, rising inflation and interest rates, continued market volatility and decreased consumer confidence. These risks are not exhaustive, Aptevo faces known and unknown risks. Additional risks and factors that may affect results are set forth in Aptevo's filings with the Securities and Exchange Commission, including its Annual Report on Form 10-K for the fiscal year ended December 31, 2024, and its subsequent reports on Form 10-Q and current reports on Form 8-K. The foregoing sets forth many, but not all, of the factors that could cause actual results to differ from Aptevo's expectations in any forward-looking statement. Any forward-looking statement speaks only as of the date of this press release, and, except as required by law, Aptevo does not assume any obligation to update any forward-looking statement to reflect new information, events, or circumstances.
CONTACT:
Miriam Weber Miller
Head, Investor Relations & Corporate Communications
Aptevo Therapeutics
Email: [email protected] or [email protected]
Phone: 206-859-6628
SOURCE: Aptevo Therapeutics
View the original press release on ACCESS Newswire
G.Stevens--AMWN