
-
 Spotify says piracy activists hacked its music catalogue
Spotify says piracy activists hacked its music catalogue
-
Winter Olympics organisers resolve snow problem at ski site

-
 Fuming Denmark summons US ambassador over Greenland envoy
Fuming Denmark summons US ambassador over Greenland envoy
-
UK's street artist Banksy unveils latest mural in London

-
 Rugby players lose order challenge in brain injury claim
Rugby players lose order challenge in brain injury claim
-
UK singer Chris Rea dies at 74, days before Christmas

-
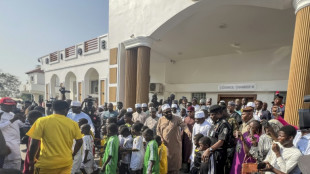 Last of kidnapped Nigerian pupils handed over, government says
Last of kidnapped Nigerian pupils handed over, government says
-
Zambia strike late to hold Mali in AFCON opener

-
 Outcry follows CBS pulling program on prison key to Trump deportations
Outcry follows CBS pulling program on prison key to Trump deportations
-
Sri Lanka cyclone caused $4.1 bn damage: World Bank

-
 Billionaire Ellison offers personal guarantee for son's bid for Warner Bros
Billionaire Ellison offers personal guarantee for son's bid for Warner Bros
-
Tech stocks lead Wall Street higher, gold hits fresh record

-
 Telefonica to shed around 5,500 jobs in Spain
Telefonica to shed around 5,500 jobs in Spain
-
McCullum wants to stay as England coach despite Ashes drubbing

-
 EU slams China dairy duties as 'unjustified'
EU slams China dairy duties as 'unjustified'
-
Italy fines Apple nearly 100 mn euros over app privacy feature

-
 America's Cup switches to two-year cycle
America's Cup switches to two-year cycle
-
Jesus could start for Arsenal in League Cup, says Arteta

-
 EU to probe Czech aid for two nuclear units
EU to probe Czech aid for two nuclear units
-
Strauss says sacking Stokes and McCullum will not solve England's Ashes woes

-
 Noel takes narrow lead after Alta Badia slalom first run
Noel takes narrow lead after Alta Badia slalom first run
-
Stocks diverge as rate hopes rise, AI fears ease

-
 Man City players face Christmas weigh-in as Guardiola issues 'fatty' warning
Man City players face Christmas weigh-in as Guardiola issues 'fatty' warning
-
German Christmas markets hit by flood of fake news

-
 Liverpool fear Isak has broken leg: reports
Liverpool fear Isak has broken leg: reports
-
West Indies captain says he 'let the team down' in New Zealand Tests

-
 Thailand says Cambodia agrees to border talks after ASEAN meet
Thailand says Cambodia agrees to border talks after ASEAN meet
-
Alleged Bondi shooters conducted 'tactical' training in countryside, Australian police say

-
 Swiss court to hear landmark climate case against cement giant
Swiss court to hear landmark climate case against cement giant
-
Knicks' Brunson scores 47, Bulls edge Hawks epic

-
 Global nuclear arms control under pressure in 2026
Global nuclear arms control under pressure in 2026
-
Asian markets rally with Wall St as rate hopes rise, AI fears ease

-
 Jailed Malaysian ex-PM Najib loses bid for house arrest
Jailed Malaysian ex-PM Najib loses bid for house arrest
-
Banned film exposes Hong Kong's censorship trend, director says

-
 Duffy, Patel force West Indies collapse as NZ close in on Test series win
Duffy, Patel force West Indies collapse as NZ close in on Test series win
-
Australian state pushes tough gun laws, 'terror symbols' ban after shooting

-
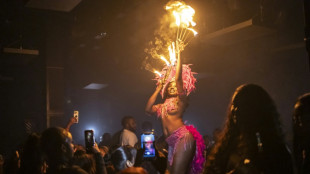 A night out on the town during Nigeria's 'Detty December'
A night out on the town during Nigeria's 'Detty December'
-
US in 'pursuit' of third oil tanker in Caribbean: official

-
 CO2 soon to be buried under North Sea oil platform
CO2 soon to be buried under North Sea oil platform
-
Steelers edge Lions as Bears, 49ers reach playoffs

-
 India's Bollywood counts costs as star fees squeeze profits
India's Bollywood counts costs as star fees squeeze profits
-
McCullum admits errors in Ashes preparations as England look to salvage pride

-
 Pets, pedis and peppermints: When the diva is a donkey
Pets, pedis and peppermints: When the diva is a donkey
-
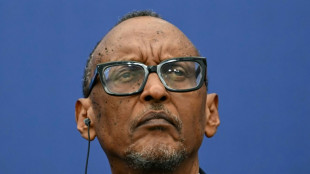
'A den of bandits': Rwanda closes thousands of evangelical churches
-
 Southeast Asia bloc meets to press Thailand, Cambodia on truce
Southeast Asia bloc meets to press Thailand, Cambodia on truce
-
As US battles China on AI, some companies choose Chinese

-
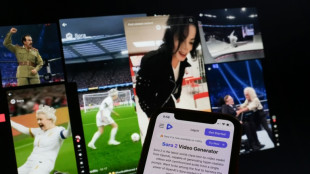 AI resurrections of dead celebrities amuse and rankle
AI resurrections of dead celebrities amuse and rankle
-
SMX and Partners Push Gold Compliance Out of the Back Office and Into the Supply Chain

-
 Jonathan Blum Launches Next-Generation Real Estate Brand Empowering Smart Investors in South Florida
Jonathan Blum Launches Next-Generation Real Estate Brand Empowering Smart Investors in South Florida
-
International Symposium on Archaeology & The Bible IX to Feature Esteemed Voices in Biblical Studies and Archaeology


Hemogenyx Pharmaceuticals PLC - Third Patient Treated with HG-CT-1 CAR-T Therapy
Hemogenyx Pharmaceuticals plc
("Hemogenyx Pharmaceuticals" or the "Company")
Third Patient Treated with HG-CT-1 CAR-T Therapy and Completion of First Adult Dose Cohort
LONDON, UNITED KINGDOM / ACCESS Newswire / August 15, 2025 / Hemogenyx Pharmaceuticals plc (LSE:HEMO) is pleased to announce that the third patient has been successfully treated as part of the Company's ongoing Phase I clinical trial of HG-CT-1, its proprietary CAR-T cell therapy for relapsed or refractory acute myeloid leukemia ("R/R AML") in adults.
Treatment of this patient, the final participant in the first adult dose cohort, was made possible after the Company secured special permission from the U.S. Food and Drug Administration (FDA) to proceed under exceptional circumstances. This regulatory clearance reflects the Company's ability to navigate complex clinical challenges to ensure eligible patients can access potentially life-saving therapies.
Completion of the first adult dose cohort, which received the lowest dose of HG-CT-1, represents a pivotal milestone in the Company's trial. If no dose-limiting toxicities are observed, Hemogenyx Pharmaceuticals will:
Advance to the second adult dose cohort at twice the initial dose, and
Initiate recruitment for the pediatric arm of the trial, addressing a critical unmet need in childhood AML.
The Phase I trial is a dose-escalation study designed to assess safety and collect data on key secondary endpoints, including anti-leukemic activity, overall survival, progression-free survival, and duration of response. The Company is pleased to report that the first two patients treated with HG-CT-1 remain alive at six months and three months post-treatment, respectively.
Dr. Vladislav Sandler, CEO & Co-Founder of Hemogenyx Pharmaceuticals, commented:
"Completing the first adult dose cohort is a major achievement in our Phase I trial of HG-CT-1. I am proud of our team's persistence in working with the FDA to obtain the necessary clearance to treat this patient. With this milestone reached, we are well positioned to move into the higher-dose cohort and to open our pediatric arm, advancing our mission to deliver transformative therapies for AML patients of all ages."
Market Abuse Regulation (MAR) Disclosure
Certain information contained in this announcement would have been inside information for the purposes of Article 7 of Regulation No 596/2014 (as it forms part of UK domestic law by virtue of the European Union (Withdrawal) Act 2018) until the release of this announcement. The person responsible for arranging for the release of this announcement on behalf of Hemogenyx Pharmaceuticals plc is Dr Vladislav Sandler, Chief Executive Officer & Co-Founder.
Enquiries:
Hemogenyx Pharmaceuticals plc | |
Dr Vladislav Sandler, Chief Executive Officer & Co-Founder | |
Peter Redmond, Director | |
SP Angel Corporate Finance LLP | Tel: +44 (0)20 3470 0470 |
Matthew Johnson, Vadim Alexandre, Adam Cowl | |
Peterhouse Capital Limited | Tel: +44 (0)20 7469 0930 |
Lucy Williams, Duncan Vasey, Charles Goodfellow |
About Hemogenyx Pharmaceuticals plc
Hemogenyx Pharmaceuticals is a publicly traded company (LSE:HEMO) headquartered in London, with its US operating subsidiaries, Hemogenyx Pharmaceuticals LLC and Immugenyx LLC, located in New York City.
The Company is a clinical-stage biopharmaceutical group developing new medicines and treatments to treat blood and autoimmune diseases. Hemogenyx Pharmaceuticals is developing several distinct and complementary product candidates, as well as platform technologies that it uses as engines for novel product development.
This information is provided by RNS, the news service of the London Stock Exchange. RNS is approved by the Financial Conduct Authority to act as a Primary Information Provider in the United Kingdom. Terms and conditions relating to the use and distribution of this information may apply. For further information, please contact [email protected] or visit www.rns.com.
SOURCE: Hemogenyx Pharmaceuticals PLC
View the original press release on ACCESS Newswire
P.Martin--AMWN