
-
 Spotify says piracy activists hacked its music catalogue
Spotify says piracy activists hacked its music catalogue
-
Winter Olympics organisers resolve snow problem at ski site

-
 Fuming Denmark summons US ambassador over Greenland envoy
Fuming Denmark summons US ambassador over Greenland envoy
-
UK's street artist Banksy unveils latest mural in London

-
 Rugby players lose order challenge in brain injury claim
Rugby players lose order challenge in brain injury claim
-
UK singer Chris Rea dies at 74, days before Christmas

-
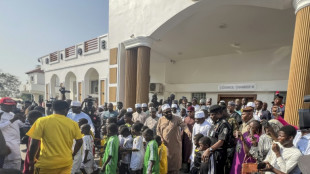 Last of kidnapped Nigerian pupils handed over, government says
Last of kidnapped Nigerian pupils handed over, government says
-
Zambia strike late to hold Mali in AFCON opener

-
 Outcry follows CBS pulling program on prison key to Trump deportations
Outcry follows CBS pulling program on prison key to Trump deportations
-
Sri Lanka cyclone caused $4.1 bn damage: World Bank

-
 Billionaire Ellison offers personal guarantee for son's bid for Warner Bros
Billionaire Ellison offers personal guarantee for son's bid for Warner Bros
-
Tech stocks lead Wall Street higher, gold hits fresh record

-
 Telefonica to shed around 5,500 jobs in Spain
Telefonica to shed around 5,500 jobs in Spain
-
McCullum wants to stay as England coach despite Ashes drubbing

-
 EU slams China dairy duties as 'unjustified'
EU slams China dairy duties as 'unjustified'
-
Italy fines Apple nearly 100 mn euros over app privacy feature

-
 America's Cup switches to two-year cycle
America's Cup switches to two-year cycle
-
Jesus could start for Arsenal in League Cup, says Arteta

-
 EU to probe Czech aid for two nuclear units
EU to probe Czech aid for two nuclear units
-
Strauss says sacking Stokes and McCullum will not solve England's Ashes woes

-
 Noel takes narrow lead after Alta Badia slalom first run
Noel takes narrow lead after Alta Badia slalom first run
-
Stocks diverge as rate hopes rise, AI fears ease

-
 Man City players face Christmas weigh-in as Guardiola issues 'fatty' warning
Man City players face Christmas weigh-in as Guardiola issues 'fatty' warning
-
German Christmas markets hit by flood of fake news

-
 Liverpool fear Isak has broken leg: reports
Liverpool fear Isak has broken leg: reports
-
West Indies captain says he 'let the team down' in New Zealand Tests

-
 Thailand says Cambodia agrees to border talks after ASEAN meet
Thailand says Cambodia agrees to border talks after ASEAN meet
-
Alleged Bondi shooters conducted 'tactical' training in countryside, Australian police say

-
 Swiss court to hear landmark climate case against cement giant
Swiss court to hear landmark climate case against cement giant
-
Knicks' Brunson scores 47, Bulls edge Hawks epic

-
 Global nuclear arms control under pressure in 2026
Global nuclear arms control under pressure in 2026
-
Asian markets rally with Wall St as rate hopes rise, AI fears ease

-
 Jailed Malaysian ex-PM Najib loses bid for house arrest
Jailed Malaysian ex-PM Najib loses bid for house arrest
-
Banned film exposes Hong Kong's censorship trend, director says

-
 Duffy, Patel force West Indies collapse as NZ close in on Test series win
Duffy, Patel force West Indies collapse as NZ close in on Test series win
-
Australian state pushes tough gun laws, 'terror symbols' ban after shooting

-
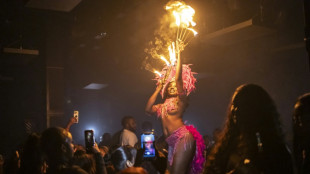 A night out on the town during Nigeria's 'Detty December'
A night out on the town during Nigeria's 'Detty December'
-
US in 'pursuit' of third oil tanker in Caribbean: official

-
 CO2 soon to be buried under North Sea oil platform
CO2 soon to be buried under North Sea oil platform
-
Steelers edge Lions as Bears, 49ers reach playoffs

-
 India's Bollywood counts costs as star fees squeeze profits
India's Bollywood counts costs as star fees squeeze profits
-
McCullum admits errors in Ashes preparations as England look to salvage pride

-
 Pets, pedis and peppermints: When the diva is a donkey
Pets, pedis and peppermints: When the diva is a donkey
-
'A den of bandits': Rwanda closes thousands of evangelical churches
-
 Southeast Asia bloc meets to press Thailand, Cambodia on truce
Southeast Asia bloc meets to press Thailand, Cambodia on truce
-
As US battles China on AI, some companies choose Chinese

-
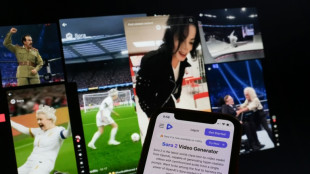 AI resurrections of dead celebrities amuse and rankle
AI resurrections of dead celebrities amuse and rankle
-
SMX and Partners Push Gold Compliance Out of the Back Office and Into the Supply Chain

-
 Jonathan Blum Launches Next-Generation Real Estate Brand Empowering Smart Investors in South Florida
Jonathan Blum Launches Next-Generation Real Estate Brand Empowering Smart Investors in South Florida
-
International Symposium on Archaeology & The Bible IX to Feature Esteemed Voices in Biblical Studies and Archaeology


CNS Pharmaceuticals Reports Second Quarter 2025 Financial Results and Provides Corporate Update
Strong cash position of $12.1 million expected to fund operations into the second half of 2026
Driving lead program, TPI 287, towards Phase 2 study for treatment of glioblastoma multiforme (GBM)
HOUSTON, TX / ACCESS Newswire / August 15, 2025 / CNS Pharmaceuticals, Inc. (NASDAQ:CNSP) ("CNS" or the "Company"), a biopharmaceutical company specializing in the development of novel treatments for primary and metastatic cancers in the brain and central nervous system, today reported its financial results for the second quarter ended June 30, 2025 and provided a corporate update.
"Every decision we make is grounded in our science and driven by a sense of urgency and responsibility to the patients and families counting on us for better outcomes. We are making excellent progress in our development strategy for TPI 287, working rapidly toward commencing a Phase 2 study for the treatment of GBM in the first half of 2026. Looking ahead, we are continuing to leverage our operational experience and global infrastructure to support TPI 287 while working to get our trial up and running and begin dosing patients with this exciting drug candidate," commented John Climaco, CEO of CNS Pharmaceuticals.
TPI 287: Late Stage, Novel Blood Brain Barrier Permeable Abeotaxane for Treatment of Brain Malignancies
TPI 287 is an abeotaxane and has the same mechanism of action as other taxanes, e.g. paclitaxel (Taxol®) and docetaxel, in which it stabilizes microtubules and inhibits cell division, causing apoptosis and cell death. While most taxanes are substrates for multi-drug resistant transporters, which maintain the blood brain barrier (BBB), TPI 287's clinical data suggest it has the potential to cross the BBB and treat CNS tumors. In a Phase 1 trial treating glioblastoma patients with TPI 287 in combination with bevacizumab (Avastin®), the efficacy data included 3 Complete Responses and 9 Partial Responses out of 23 patients evaluable.
As previously announced, TPI 287 was granted Orphan Drug Designation from the U.S. Food and Drug Administration (FDA) for the treatment of gliomas, pediatric neuroblastoma, and progressive supranuclear palsy.
CNS Pharmaceuticals plans to engage the FDA on the design of a study potentially focused on the registration of TPI 287 in recurrent GBM in 2025.
Summary of Financial Results for the Second Quarter 2025
The net loss for the three months ended June 30, 2025 was approximately $2.4 million compared to approximately $2.5 million for the comparable period in 2024. The change in net loss is largely attributable to declining trial costs on the Berubicin trial.
The Company reported research and development expenses of $1.2 million for the three months ended June 30, 2025 compared to approximately $1.1 million for the comparable period in 2024. The increase in research and development expenses during the period were mainly attributed to declining trial costs on the Berubicin trial offset by expenditures preparing for a TPI 287 trial including drug manufacturing.
General and administrative expense was approximately $1.2 million for the three months ended June 30, 2025 compared to approximately $1.4 million for the comparable period in 2024. The decrease in general and administrative expense is attributable to decreases of approximately $36,000 in legal and professional expenses, $19,000 in travel expenses, $195,000 in stock-based compensation, which were offset by increases of approximately $51,000 in compensation expense and $29,000 in insurance expense.
As of June 30, 2025, the Company had cash of approximately $12.1 million. The Company believes that the cash on hand as of June 30, 2025 is sufficient to fund planned operations into the second half of 2026.
About CNS Pharmaceuticals, Inc.
CNS Pharmaceuticals is a clinical-stage pharmaceutical company developing a pipeline of anti-cancer drug candidates for the treatment of primary and metastatic cancers of the brain and central nervous system.
The Company's drug candidate TPI 287 is an abeotaxane, which stabilizes microtubules and inhibits cell division, causing apoptosis and cell death. The initial clinical efficacy data suggest TPI 287 has the potential to cross the blood-brain barrier and treat CNS tumors. TPI 287 also has been tested in over 350 patients in clinical trials as a monotherapy and in combination with bevacizumab for the treatment of a range of diseases or conditions, including recurrent glioblastoma, recurrent neuroblastoma and medulloblastoma, advanced malignancies, advanced unresectable pancreatic cancer, metastatic melanoma, and breast cancer metastatic to the brain. To date TPI 287 appears have both an excellent safety profile and high tolerability among patients.
For more information, please visit www.CNSPharma.com, and connect with the Company on X, Facebook, and LinkedIn.
Forward-Looking Statements
Some of the statements in this press release are forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, Section 21E of the Securities Exchange Act of 1934 and the Private Securities Litigation Reform Act of 1995, which involve risks and uncertainties. Forward-looking statements in this release include, without limitation, the timing of the start of a trial of TPI 287and the Company's cash runway. These statements relate to future events, future expectations, plans and prospects. Although CNS believes the expectations reflected in such forward-looking statements are reasonable as of the date made, expectations may prove to have been materially different from the results expressed or implied by such forward-looking statements. CNS has attempted to identify forward-looking statements by terminology including ''believes,'' ''estimates,'' ''anticipates,'' ''expects,'' ''plans,'' ''projects,'' ''intends,'' ''potential,'' ''may,'' ''could,'' ''might,'' ''will,'' ''should,'' ''approximately'' or other words that convey uncertainty of future events or outcomes to identify these forward-looking statements. These statements are only predictions and involve known and unknown risks, uncertainties and other factors, including market and other conditions and those discussed under Item 1A. "Risk Factors" in CNS's most recently filed Form 10-K filed with the Securities and Exchange Commission ("SEC") and updated from time to time in its Form 10-Q filings and in its other public filings with the SEC. Any forward-looking statements contained in this press release speak only as of its date. CNS undertakes no obligation to update any forward-looking statements contained in this press release to reflect events or circumstances occurring after its date or to reflect the occurrence of unanticipated events, except as required by law.
CONTACTS:
Investor Relations Contact
JTC Team, LLC
Jenene Thomas
908.824.0775
[email protected]
SOURCE: CNS Pharmaceuticals, Inc.
View the original press release on ACCESS Newswire
T.Ward--AMWN