
-
 UK singer Chris Rea dies at 74, days before Christmas
UK singer Chris Rea dies at 74, days before Christmas
-
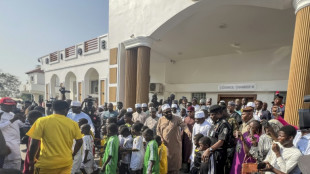
Last of kidnapped Nigerian pupils handed over, government says
-
 Zambia strike late to hold Mali in AFCON opener
Zambia strike late to hold Mali in AFCON opener
-
Outcry follows CBS pulling program on prison key to Trump deportations

-
 Sri Lanka cyclone caused $4.1 bn damage: World Bank
Sri Lanka cyclone caused $4.1 bn damage: World Bank
-
Billionaire Ellison offers personal guarantee for son's bid for Warner Bros

-
 Tech stocks lead Wall Street higher, gold hits fresh record
Tech stocks lead Wall Street higher, gold hits fresh record
-
Telefonica to shed around 5,500 jobs in Spain

-
 McCullum wants to stay as England coach despite Ashes drubbing
McCullum wants to stay as England coach despite Ashes drubbing
-
EU slams China dairy duties as 'unjustified'

-
 Italy fines Apple nearly 100 mn euros over app privacy feature
Italy fines Apple nearly 100 mn euros over app privacy feature
-
America's Cup switches to two-year cycle

-
 Jesus could start for Arsenal in League Cup, says Arteta
Jesus could start for Arsenal in League Cup, says Arteta
-
EU to probe Czech aid for two nuclear units

-
 Strauss says sacking Stokes and McCullum will not solve England's Ashes woes
Strauss says sacking Stokes and McCullum will not solve England's Ashes woes
-
Noel takes narrow lead after Alta Badia slalom first run

-
 Stocks diverge as rate hopes rise, AI fears ease
Stocks diverge as rate hopes rise, AI fears ease
-
Man City players face Christmas weigh-in as Guardiola issues 'fatty' warning

-
 German Christmas markets hit by flood of fake news
German Christmas markets hit by flood of fake news
-
Liverpool fear Isak has broken leg: reports

-
 West Indies captain says he 'let the team down' in New Zealand Tests
West Indies captain says he 'let the team down' in New Zealand Tests
-
Thailand says Cambodia agrees to border talks after ASEAN meet

-
 Alleged Bondi shooters conducted 'tactical' training in countryside, Australian police say
Alleged Bondi shooters conducted 'tactical' training in countryside, Australian police say
-
Swiss court to hear landmark climate case against cement giant

-
 Knicks' Brunson scores 47, Bulls edge Hawks epic
Knicks' Brunson scores 47, Bulls edge Hawks epic
-
Global nuclear arms control under pressure in 2026

-
 Asian markets rally with Wall St as rate hopes rise, AI fears ease
Asian markets rally with Wall St as rate hopes rise, AI fears ease
-
Jailed Malaysian ex-PM Najib loses bid for house arrest

-
 Banned film exposes Hong Kong's censorship trend, director says
Banned film exposes Hong Kong's censorship trend, director says
-
Duffy, Patel force West Indies collapse as NZ close in on Test series win

-
 Australian state pushes tough gun laws, 'terror symbols' ban after shooting
Australian state pushes tough gun laws, 'terror symbols' ban after shooting
-
A night out on the town during Nigeria's 'Detty December'
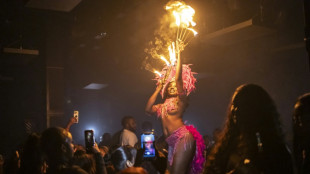
-
 US in 'pursuit' of third oil tanker in Caribbean: official
US in 'pursuit' of third oil tanker in Caribbean: official
-
CO2 soon to be buried under North Sea oil platform

-
 Steelers edge Lions as Bears, 49ers reach playoffs
Steelers edge Lions as Bears, 49ers reach playoffs
-
India's Bollywood counts costs as star fees squeeze profits

-
 McCullum admits errors in Ashes preparations as England look to salvage pride
McCullum admits errors in Ashes preparations as England look to salvage pride
-
Pets, pedis and peppermints: When the diva is a donkey

-
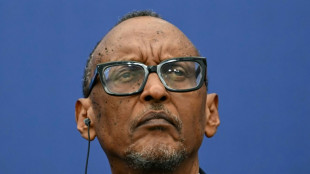 'A den of bandits': Rwanda closes thousands of evangelical churches
'A den of bandits': Rwanda closes thousands of evangelical churches
-
Southeast Asia bloc meets to press Thailand, Cambodia on truce

-
 As US battles China on AI, some companies choose Chinese
As US battles China on AI, some companies choose Chinese
-
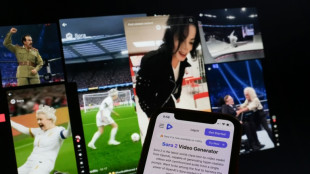
AI resurrections of dead celebrities amuse and rankle
-
 Informa Markets Health and Nutrition Brands Highlight Strategic Partnerships, Championing Voices Across Food, Nutrition, Health and Wellness
Informa Markets Health and Nutrition Brands Highlight Strategic Partnerships, Championing Voices Across Food, Nutrition, Health and Wellness
-
SMX Mission To Provide Gold Verified Identity Advances With Two New Industry Alliances

-
 Parallel Society Reveals Lineup for 2026 Lisbon Edition - A Cross-Genre Mashup of Cultural and Tech Pioneers
Parallel Society Reveals Lineup for 2026 Lisbon Edition - A Cross-Genre Mashup of Cultural and Tech Pioneers
-
Ai4 2026 Announces Dynamic Keynote Panel Featuring Geoffrey Hinton, Fei‑Fei Li & Andrew Ng

-
 NESR Becomes First Oilfield Services Company to Commission Original Artwork Created from Recycled Produced Water
NESR Becomes First Oilfield Services Company to Commission Original Artwork Created from Recycled Produced Water
-
SMX Strikes Joint Initiative with FinGo & Bougainville Refinery Ltd to Deliver Verifiable Identification for Trillion Dollar Gold Market

-
 Blue Gold and Trust Stamp Execute Strategic LOI to Develop Biometric, Passwordless Wallet Infrastructure for Gold-Backed Digital Assets
Blue Gold and Trust Stamp Execute Strategic LOI to Develop Biometric, Passwordless Wallet Infrastructure for Gold-Backed Digital Assets
-
SK tes Announces Grand Opening of New Shannon Facility, Marking a Milestone for Sustainable Technology in Ireland


President Trump's Marijuana Fix? DEA’s Program Exposed: Promises Made, Promises Not Kept
"As President Trump weighs marijuana rescheduling in the coming weeks, the DEA's track record reveals years of mismanagement, empty promises, and patients left waiting," stated MMJ CEO Duane Boise.
WASHINGTON, D.C. / ACCESS Newswire / August 17, 2025 / President Trump's decision to potentially reschedule marijuana is the first serious step in decades to break through the bureaucratic logjam that has kept patients and scientists waiting. For years, the DEA has promised a functional marijuana research program. For years, those promises have been broken.

DEA's Promises on Paper
In its Final Rule (85 Fed. Reg. 82333, Dec. 18, 2020), the DEA pledged that marijuana manufacturing licenses would be tied to FDA science and genuine medical research. The agency declared:
"DEA will evaluate each application… to determine whether the applicant's proposed activities… will promote medical research."
"DEA will require applicants to demonstrate bona fide supply agreements with researchers authorized by the FDA to conduct clinical research."
"DEA's goal is to ensure that the United States has a sufficient supply of marihuana to meet the legitimate medical, scientific, and research needs of the United States."
These were the promises.
The Harsh Reality
But DEA's actions told a different story:
Licenses granted to companies with no FDA INDs, no clinical trial protocols, and no scientific foundation.
No bona fide supply agreements with FDA-authorized researchers - a direct violation of DEA's own Final Rule.
Four years later, there is still no adequate, federally compliant supply of cannabis for clinical trials, leaving patients with Huntington's Disease, Multiple Sclerosis, and other conditions waiting.
The only company that meets DEA's own published criteria - MMJ BioPharma, with two FDA-filed INDs and an Orphan Drug Designation - has been stalled while non-scientific registrants received licenses.
Promises Made, Promises Not Kept
DEA's marijuana program has been a case study in mismanagement:
Rules written, then ignored.
Patients promised access, then denied it.
Science promised priority, then sidelined.
President Trump's possible rescheduling decision shines a spotlight on this failure. By moving marijuana to Schedule III, he has forced the issue back into the open and made clear that real reform will require DEA accountability.
The Path Forward
Patients deserve more than empty promises. They deserve consistent, reproducible, FDA-approved medicines. They deserve agencies that follow their own rules. And they deserve leadership that keeps its word.
With rescheduling now on the table, it is time to hold DEA to account for the gap between its promises and its performance. President Trump has taken the first step - now the DEA must be compelled to deliver.
MMJ is represented by attorney Megan Sheehan.
CONTACT:
Madison Hisey
[email protected]
203-231-8583
SOURCE: MMJ International Holdings
View the original press release on ACCESS Newswire
X.Karnes--AMWN