
-
 UK singer Chris Rea dies at 74, days before Christmas
UK singer Chris Rea dies at 74, days before Christmas
-
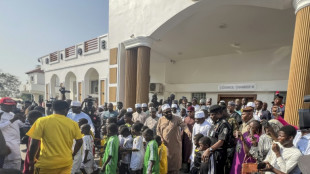
Last of kidnapped Nigerian pupils handed over, government says
-
 Zambia strike late to hold Mali in AFCON opener
Zambia strike late to hold Mali in AFCON opener
-
Outcry follows CBS pulling program on prison key to Trump deportations

-
 Sri Lanka cyclone caused $4.1 bn damage: World Bank
Sri Lanka cyclone caused $4.1 bn damage: World Bank
-
Billionaire Ellison offers personal guarantee for son's bid for Warner Bros

-
 Tech stocks lead Wall Street higher, gold hits fresh record
Tech stocks lead Wall Street higher, gold hits fresh record
-
Telefonica to shed around 5,500 jobs in Spain

-
 McCullum wants to stay as England coach despite Ashes drubbing
McCullum wants to stay as England coach despite Ashes drubbing
-
EU slams China dairy duties as 'unjustified'

-
 Italy fines Apple nearly 100 mn euros over app privacy feature
Italy fines Apple nearly 100 mn euros over app privacy feature
-
America's Cup switches to two-year cycle

-
 Jesus could start for Arsenal in League Cup, says Arteta
Jesus could start for Arsenal in League Cup, says Arteta
-
EU to probe Czech aid for two nuclear units

-
 Strauss says sacking Stokes and McCullum will not solve England's Ashes woes
Strauss says sacking Stokes and McCullum will not solve England's Ashes woes
-
Noel takes narrow lead after Alta Badia slalom first run

-
 Stocks diverge as rate hopes rise, AI fears ease
Stocks diverge as rate hopes rise, AI fears ease
-
Man City players face Christmas weigh-in as Guardiola issues 'fatty' warning

-
 German Christmas markets hit by flood of fake news
German Christmas markets hit by flood of fake news
-
Liverpool fear Isak has broken leg: reports

-
 West Indies captain says he 'let the team down' in New Zealand Tests
West Indies captain says he 'let the team down' in New Zealand Tests
-
Thailand says Cambodia agrees to border talks after ASEAN meet

-
 Alleged Bondi shooters conducted 'tactical' training in countryside, Australian police say
Alleged Bondi shooters conducted 'tactical' training in countryside, Australian police say
-
Swiss court to hear landmark climate case against cement giant

-
 Knicks' Brunson scores 47, Bulls edge Hawks epic
Knicks' Brunson scores 47, Bulls edge Hawks epic
-
Global nuclear arms control under pressure in 2026

-
 Asian markets rally with Wall St as rate hopes rise, AI fears ease
Asian markets rally with Wall St as rate hopes rise, AI fears ease
-
Jailed Malaysian ex-PM Najib loses bid for house arrest

-
 Banned film exposes Hong Kong's censorship trend, director says
Banned film exposes Hong Kong's censorship trend, director says
-
Duffy, Patel force West Indies collapse as NZ close in on Test series win

-
 Australian state pushes tough gun laws, 'terror symbols' ban after shooting
Australian state pushes tough gun laws, 'terror symbols' ban after shooting
-
A night out on the town during Nigeria's 'Detty December'
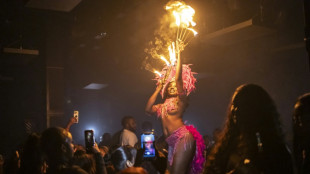
-
 US in 'pursuit' of third oil tanker in Caribbean: official
US in 'pursuit' of third oil tanker in Caribbean: official
-
CO2 soon to be buried under North Sea oil platform

-
 Steelers edge Lions as Bears, 49ers reach playoffs
Steelers edge Lions as Bears, 49ers reach playoffs
-
India's Bollywood counts costs as star fees squeeze profits

-
 McCullum admits errors in Ashes preparations as England look to salvage pride
McCullum admits errors in Ashes preparations as England look to salvage pride
-
Pets, pedis and peppermints: When the diva is a donkey

-
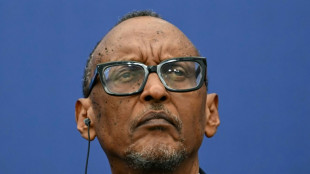 'A den of bandits': Rwanda closes thousands of evangelical churches
'A den of bandits': Rwanda closes thousands of evangelical churches
-
Southeast Asia bloc meets to press Thailand, Cambodia on truce

-
 As US battles China on AI, some companies choose Chinese
As US battles China on AI, some companies choose Chinese
-
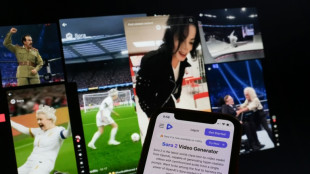
AI resurrections of dead celebrities amuse and rankle
-
 Informa Markets Health and Nutrition Brands Highlight Strategic Partnerships, Championing Voices Across Food, Nutrition, Health and Wellness
Informa Markets Health and Nutrition Brands Highlight Strategic Partnerships, Championing Voices Across Food, Nutrition, Health and Wellness
-
SMX Mission To Provide Gold Verified Identity Advances With Two New Industry Alliances

-
 Parallel Society Reveals Lineup for 2026 Lisbon Edition - A Cross-Genre Mashup of Cultural and Tech Pioneers
Parallel Society Reveals Lineup for 2026 Lisbon Edition - A Cross-Genre Mashup of Cultural and Tech Pioneers
-
Ai4 2026 Announces Dynamic Keynote Panel Featuring Geoffrey Hinton, Fei‑Fei Li & Andrew Ng

-
 NESR Becomes First Oilfield Services Company to Commission Original Artwork Created from Recycled Produced Water
NESR Becomes First Oilfield Services Company to Commission Original Artwork Created from Recycled Produced Water
-
SMX Strikes Joint Initiative with FinGo & Bougainville Refinery Ltd to Deliver Verifiable Identification for Trillion Dollar Gold Market

-
 Blue Gold and Trust Stamp Execute Strategic LOI to Develop Biometric, Passwordless Wallet Infrastructure for Gold-Backed Digital Assets
Blue Gold and Trust Stamp Execute Strategic LOI to Develop Biometric, Passwordless Wallet Infrastructure for Gold-Backed Digital Assets
-
SK tes Announces Grand Opening of New Shannon Facility, Marking a Milestone for Sustainable Technology in Ireland


Genflow Biosciences PLC Announces Company Update on Dog Trials
Genflow Advances World's First Longevity Gene Therapy Trial in Dogs with No Adverse Effects Reported
LONDON, UK / ACCESS Newswire / August 18, 2025 / Genflow Biosciences Plc (LSE:GENF)(OTCQB:GENFF) ("Genflow" or "the Company"), the only publicly listed longevity company in Europe, provides an update on its Dog Aging (GF-1004) study, a proof-of-concept clinical trial evaluating the safety and efficacy of its proprietary SIRT6-centenarian gene therapy for age-related decline in elderly dogs.
The randomized, controlled trial, conducted with CRO Syngene, involves 28 beagles aged 10+ years. In March, recipients received three different modalities of SIRT6 gene therapy with no ill or adverse effects, while controls remain untreated. The dogs will now enter a five-month follow-up period expected to conclude in January 2026.
The strategic goal of this proof-of-concept trial is to generate compelling preclinical data that will enable a licensing agreement with a leading Animal Health company. GF-1004 is a first-in-class gene therapy candidate targeting fundamental aging mechanisms shared by both dogs and humans. By intervening at the mitochondrial and epigenetic levels, GF-1004 offers the potential to not only extend lifespan, but also improve the quality of life in companion animals, a rapidly growing, high-value market segment.
Study objectives are to:
1. Confirm feasibility of GF-1004 administration in the target population.
2. Validate safety and efficacy at the proposed administered dose.
3. Demonstrate multi-dimensional benefits relevant to both clinical outcomes and consumer appeal, including:
Reduced biological age (GRIM methylation clock)
Increased muscle strength and mass
Enhanced mitochondrial function
Improved coat quality
Better overall health metrics
Translational Potential
Since dogs and humans share conserved aging pathways, success in this trial will provide a strong foundation for expansion into human health applications, unlocking additional markets in longevity therapeutics.
Dr. Eric Leire, CEO of Genflow, commented: "Now that all dogs have received the treatment without any adverse events, we've taken an important step in confirming the treatment's safety profile. We are fully focused on advancing GF-1004 to secure a partnership with an animal health company, combining scientific and commercial expertise to pave the way for a new class of therapeutic medicines for dogs. Our shared goal is to extend the healthspan of dogs, and we believe this study will demonstrate the safety and potential efficacy of GF-1004 while opening the door to broader applications of our proprietary platform in age-related diseases in both companion animals and, ultimately, in humans."
Contacts
Genflow Biosciences | Harbor Access |
Dr Eric Leire, CEO | Jonathan Paterson, Investor Relations |
+32-477-495-881 | +1 475 477 9401 |
Corporate Brokers |
Capital Plus Partners Ltd |
Jon Critchley, +44 207 432 0501 |
About Genflow Biosciences
Founded in 2020, Genflow Biosciences Plc. (LSE:GENF) (OTCQB:GENFF), a biotechnology company headquartered in the UK with R&D facilities in Belgium, is pioneering gene therapies to decelerate the aging process, with the goal of promoting longer and healthier lives while mitigating the financial, emotional, and social impacts of a fast-growing aging global population. Genflow's lead compound, GF-1002, works through the delivery of a centenarian variant of the SIRT6 gene which has yielded promising preclinical results. Genflow's 12-month proof-of-concept clinical trial evaluating their SIRT6-centenarian gene therapy in aged dogs began in March 2025. Other programs planned for 2025, include a clinical trial that will explore the potential benefits of GF-1002 in treating MASH (Metabolic Dysfunction-Associated Steatohepatitis), the most prevalent chronic liver disease for which there is no effective treatments. Please visit www.genflowbio.com and follow the Company on LinkedIn and X.
DISCLAIMER
The contents of this announcement have been prepared by, and are the sole responsibility of, the Company.
This announcement may contain forward-looking statements. The forward-looking statements include, but are not limited to, statements regarding the Company's or the Directors' expectations, hopes, beliefs, intentions or strategies regarding the future. In addition, any statement that refers to projections, forecasts or other characterisations of future events or circumstances, including any underlying assumptions, is a forward-looking statement. The words "anticipate", "believe", "continue", "could", "estimate", "expect", "intend", "may", "might", "plan", "possible", "potential", "predict", "project", "seek", "should", "would" and similar expressions, or in each case their negatives, may identify forward-looking statements, but the absence of these words does not mean that a statement is not forward-looking.
Forward-looking statements include all matters that are not historical facts. Forward-looking statements are based on the current expectations and assumptions regarding the Company, the business, the economy and other future conditions. Because forward-looking statements relate to the future, by their nature, they are subject to inherent uncertainties, risks and changes in circumstances that are difficult to predict. Forward-looking statements are not guarantees of future performance and the Company's actual financial condition, actual results of operations and financial performance, and the development of the industries in which it operates or will operate, may differ materially from those made in or suggested by the forward-looking statements contained in this announcement. In addition, even if the Company's financial condition, results of operations and the development of the industries in which it operates or will operate, are consistent with the forward-looking statements contained in this announcement, those results or developments may not be indicative of financial condition, results of operations or developments in subsequent periods. Important factors that could cause actual results to differ materially from those in the forward-looking statements include regional, national or global, political, economic, social, business, technological, competitive, market and regulatory conditions.
Any forward-looking statement contained in this announcement applies only as of the date of this announcement and is expressly qualified in its entirety by these cautionary statements. Factors or events that could cause the Company's actual plans or results to differ may emerge from time to time, and it is not possible for the Company to predict all of them. The Company expressly disclaims any obligation or undertaking to release publicly any updates or revisions to any forward-looking statements contained in this announcement to reflect any change in its expectations or any change in events, conditions or circumstances on which any forward-looking statement contained in this announcement is based, unless required to do so by applicable law, the Prospectus Regulation Rules, the Listing Rules, the Disclosure Guidance and Transparency Rules of the FCA or the UK Market Abuse Regulation.
This information is provided by Reach, the non-regulatory press release distribution service of RNS, part of the London Stock Exchange. Terms and conditions relating to the use and distribution of this information may apply. For further information, please contact [email protected] or visit www.rns.com.
SOURCE: Genflow Biosciences PLC
View the original press release on ACCESS Newswire
Y.Nakamura--AMWN