
-
 Telefonica to shed around 5,500 jobs in Spain
Telefonica to shed around 5,500 jobs in Spain
-
McCullum wants to stay as England coach despite Ashes drubbing

-
 EU slams China dairy duties as 'unjustified'
EU slams China dairy duties as 'unjustified'
-
Italy fines Apple nearly 100 mn euros over app privacy feature

-
 America's Cup switches to two-year cycle
America's Cup switches to two-year cycle
-
Jesus could start for Arsenal in League Cup, says Arteta

-
 EU to probe Czech aid for two nuclear units
EU to probe Czech aid for two nuclear units
-
Strauss says sacking Stokes and McCullum will not solve England's Ashes woes

-
 Noel takes narrow lead after Alta Badia slalom first run
Noel takes narrow lead after Alta Badia slalom first run
-
Stocks diverge as rate hopes rise, AI fears ease

-
 Man City players face Christmas weigh-in as Guardiola issues 'fatty' warning
Man City players face Christmas weigh-in as Guardiola issues 'fatty' warning
-
German Christmas markets hit by flood of fake news

-
 Liverpool fear Isak has broken leg: reports
Liverpool fear Isak has broken leg: reports
-
West Indies captain says he 'let the team down' in New Zealand Tests

-
 Thailand says Cambodia agrees to border talks after ASEAN meet
Thailand says Cambodia agrees to border talks after ASEAN meet
-
Alleged Bondi shooters conducted 'tactical' training in countryside, Australian police say

-
 Swiss court to hear landmark climate case against cement giant
Swiss court to hear landmark climate case against cement giant
-
Knicks' Brunson scores 47, Bulls edge Hawks epic

-
 Global nuclear arms control under pressure in 2026
Global nuclear arms control under pressure in 2026
-
Asian markets rally with Wall St as rate hopes rise, AI fears ease

-
 Jailed Malaysian ex-PM Najib loses bid for house arrest
Jailed Malaysian ex-PM Najib loses bid for house arrest
-
Banned film exposes Hong Kong's censorship trend, director says

-
 Duffy, Patel force West Indies collapse as NZ close in on Test series win
Duffy, Patel force West Indies collapse as NZ close in on Test series win
-
Australian state pushes tough gun laws, 'terror symbols' ban after shooting

-
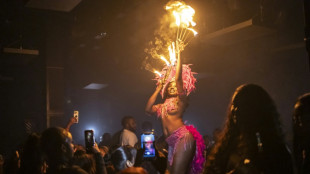 A night out on the town during Nigeria's 'Detty December'
A night out on the town during Nigeria's 'Detty December'
-
US in 'pursuit' of third oil tanker in Caribbean: official

-
 CO2 soon to be buried under North Sea oil platform
CO2 soon to be buried under North Sea oil platform
-
Steelers edge Lions as Bears, 49ers reach playoffs

-
 India's Bollywood counts costs as star fees squeeze profits
India's Bollywood counts costs as star fees squeeze profits
-
McCullum admits errors in Ashes preparations as England look to salvage pride

-
 Pets, pedis and peppermints: When the diva is a donkey
Pets, pedis and peppermints: When the diva is a donkey
-
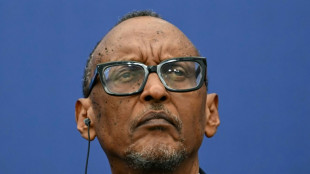
'A den of bandits': Rwanda closes thousands of evangelical churches
-
 Southeast Asia bloc meets to press Thailand, Cambodia on truce
Southeast Asia bloc meets to press Thailand, Cambodia on truce
-
As US battles China on AI, some companies choose Chinese

-
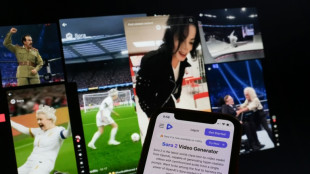 AI resurrections of dead celebrities amuse and rankle
AI resurrections of dead celebrities amuse and rankle
-
NESR Becomes First Oilfield Services Company to Commission Original Artwork Created from Recycled Produced Water
-
 SMX Strikes Joint Initiative with FinGo & Bougainville Refinery Ltd to Deliver Verifiable Identification for Trillion Dollar Gold Market
SMX Strikes Joint Initiative with FinGo & Bougainville Refinery Ltd to Deliver Verifiable Identification for Trillion Dollar Gold Market
-
Blue Gold and Trust Stamp Execute Strategic LOI to Develop Biometric, Passwordless Wallet Infrastructure for Gold-Backed Digital Assets

-
 SK tes Announces Grand Opening of New Shannon Facility, Marking a Milestone for Sustainable Technology in Ireland
SK tes Announces Grand Opening of New Shannon Facility, Marking a Milestone for Sustainable Technology in Ireland
-
FDA Officially Confirms Kava is a Food Under Federal Law

-
 Greenliant NVMe NANDrive(TM) SSDs Selected for Major Industrial, Aerospace and Mission Critical Programs
Greenliant NVMe NANDrive(TM) SSDs Selected for Major Industrial, Aerospace and Mission Critical Programs
-
World Renowned Law Firm Grant & Eisenhofer Files Class Action Lawsuit Against Canadian Banks CIBC and RBC Alleging Illegal Stock Market Manipulation of Quantum BioPharma Shares

-
 NextTrip Announces Pricing of Private Placement Financing of $3 Million
NextTrip Announces Pricing of Private Placement Financing of $3 Million
-
Namibia Critical Metals Inc. Receives Proceeds of $1,154,762 from Exercise of Warrants

-
 Shareholders Updates
Shareholders Updates
-
Applied Energetics Selected to Participate in Missile Defense Agency's Golden Dome (SHIELD) Multiple Award IDIQ Contract Vehicle

-
 Prospect Ridge Updates Diamond Drill Program at 100% Owned Camelot Copper-Gold Project in B.C.'S Cariboo Mining District
Prospect Ridge Updates Diamond Drill Program at 100% Owned Camelot Copper-Gold Project in B.C.'S Cariboo Mining District
-
The Alkaline Water Company Receives SEC Qualification of Tier 1 Regulation A Offering of Up to $10 Million

-
 Public Can Help Rid Oceans of Mines in New Freelancer Global Challenge
Public Can Help Rid Oceans of Mines in New Freelancer Global Challenge
-
Shareholders Update Report


Aspire Biopharma Announces Positive Top-Line Results from Clinical Trial of Investigational New Sublingual Aspirin Product for Treatment of Suspected Acute Myocardial Infarction (Heart Attack)
According to the NIH, Acute myocardial infarction (AMI) is a major cause of death, affecting nearly 3 million Americans each year and resulting in over a million deaths
Trial demonstrates dramatically higher and more rapid therapeutic impact compared to standard chewed aspirin tablets in clinical trial
Aspire's sublingual aspirin was safe and well-tolerated
Aspire plans to review clinical trial results with the FDA to enable a potential regulatory submission for accelerated approval
ESTERO, FL / ACCESS Newswire / August 18, 2025 / Aspire Biopharma Holdings, Inc. (Nasdaq:ASBP) ("Aspire" or the "Company"), developer of a multi-faceted patent-pending drug delivery technology, today announced positive top-line data from its recent randomized, crossover bioavailability trial to assess the safety, tolerability, pharmacokinetics (PK) and pharmacodynamics (PD) of Aspire's investigational new sublingual aspirin product compared to chewed uncoated aspirin tablets in healthy adults. Pharmacokinetics is the term that describes the four stages of absorption, distribution, metabolism, and excretion of drugs. Pharmacodynamics (sometimes described as what a drug does to the body) is the study of the biochemical, physiologic, and molecular effects of drugs on the body.
The Aspire sublingual aspirin product produced higher and more rapid mean plasma concentrations of acetylsalicylic acid (ASA, the active antiplatelet form of aspirin) compared to chewed aspirin tablets (p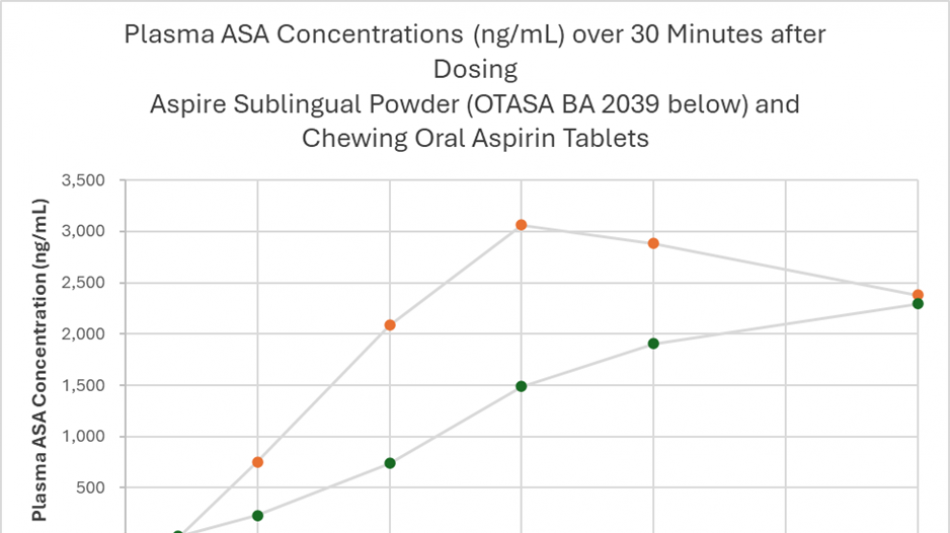
Aspire Benefits of Sublingual Aspirin Drug Delivery and Bypassing the Gut vs Standard Oral Aspirin
Rapid absorption through the blood vessels directly, bypassing first-pass metabolic processes
Faster onset of action
Sublingual route avoids exposing the drug to the harsh acidic environment of the stomach and digestive enzymes
Reduced drug-food and drug-drug interactions
Lower risk of GI irritation
Ease of administration and use in emergency situations
Clinical trial AB-101 was a randomized crossover bioavailability study of Aspire's investigational new sublingual aspirin product compared to chewed uncoated aspirin tablets. Six otherwise healthy 40 to 65-year-old subjects were administered 162 mg aspirin as a single dose in each of three treatment periods separated by two 14-day washout periods. Two different investigational sublingual powder and granule formulations (Aspire Biopharma) and chewed uncoated oral aspirin tablets (Bayer) were studied. The primary objective of the clinical trial was to evaluate the bioavailability of ASA in plasma over eight hours after dosing.
"We are extremely pleased to report these highly positive results for our sublingual aspirin formulation, providing an important validation of our drug delivery technology," said Kraig Higginson, Chief Executive Officer of Aspire. "The effectiveness of aspirin in treating a heart attack is dependent on the time it takes to deliver ASA into the bloodstream. The ability to achieve higher and more rapid ASA concentrations for patients with suspected acute MI could save lives. We would like to thank the patients, investigators and their staff for participating in the trial, and we look forward to working with regulatory agencies to discuss next steps as we work to advance this innovative therapy and improve the treatment options for patients with suspected acute MI."
Aspire is developing its investigational sublingual aspirin product for treatment of suspected acute myocardial infarction (AMI, blockage of blood flow to heart muscle causing damage or death of heart tissue - commonly known as a "heart attack"). There are an estimated 18 million Americans living with coronary artery disease with approximately 800,000 per year experiencing an AMI leading to 300,000 deaths.
Oral aspirin is FDA-approved for treatment of suspected AMI with the initial dose of 160-162.5 mg is administered as soon as an AMI is suspected.[i] In a large, multicenter study of aspirin, streptokinase, and the combination of aspirin and streptokinase in 17,187 patients with suspected AMI, aspirin treatment produced a 23 percent reduction in the risk of death from cardiovascular diseases within five weeks.[ii] Clinical practice guidelines recommend that aspirin be initiated as soon as possible with the initial dose chewed, when possible, to achieve faster onset of antiplatelet action.[iii]
Aspire's sublingual aspirin is an investigational new drug and has not been approved for marketing by FDA or any other government regulatory authority.
About Aspire Biopharma, Inc.
Aspire Biopharma has developed a patent-pending sublingual delivery technology that can deliver drugs, nutraceuticals and supplements to the body rapidly and precisely. This allows for greater effectiveness and reduced side effects by going directly to the bloodstream and avoiding the gastrointestinal tract. Aspire Biopharma's delivery technology can be applied to many different active pharmaceutical ingredients (APIs) and other bioactive substances, spanning both small and large molecule therapeutics, nutraceuticals and supplements.
For more information, please visit www.aspirebiolabs.com
Safe Harbor Statement
This press release contains "forward-looking statements" within the meaning of Section 21E of the Securities Exchange Act of 1934, as amended, and Section 27A of the Securities Act of 1933, as amended, which are intended to be covered by the "safe harbor" provisions created by those laws. Aspire's forward-looking statements include, but are not limited to, statements regarding our or our management team's expectations, hopes, beliefs, intentions or strategies regarding our future operations. In addition, any statements that refer to projections, forecasts or other characterizations of future events or circumstances, including any underlying assumptions, are forward-looking statements. The words "anticipate," "believe," "contemplate," "continue," "estimate," "expect," "intends," "may," "might," "plan," "possible," "potential," "predict," "project," "should," "will," "would," and similar expressions may identify forward-looking statements, but the absence of these words does not mean that a statement is not forward-looking. These forward-looking statements represent our views as of the date of this press release and involve a number of judgments, risks and uncertainties. We anticipate that subsequent events and developments will cause our views to change. We undertake no obligation to update forward-looking statements to reflect events or circumstances after the date they were made, whether as a result of new information, future events or otherwise, except as may be required under applicable securities laws. Accordingly, forward-looking statements should not be relied upon as representing our views as of any subsequent date. As a result of a number of known and unknown risks and uncertainties, our actual results or performance may be materially different from those expressed or implied by these forward-looking statements. Some factors that could cause actual results to differ include general market conditions, whether clinical trials demonstrate the efficacy and safety of our drug candidates to the satisfaction of regulatory authorities, or do not otherwise produce positive results which may cause us to incur additional costs or experience delays in completing, or ultimately be unable to complete the development and commercialization of our drug candidates; the clinical results for our drug candidates, which may not support further development or marketing approval; actions of regulatory agencies, which may affect the initiation, timing and progress of clinical trials and marketing approval; our ability to achieve commercial success for our drug candidates, if approved; our limited operating history and our ability to obtain additional funding for operations and to complete the development and commercialization of our drug candidates; and other risks and uncertainties set forth in "Risk Factors" in our most recent Annual Report on Form 10-K and any subsequent Quarterly Reports on Form 10-Q. In addition, statements that "we believe" and similar statements reflect our beliefs and opinions on the relevant subject. These statements are based upon information available to us as of the date of this press release, and while we believe such information forms a reasonable basis for such statements, such information may be limited or incomplete, and our statements should not be read to indicate that we have conducted an exhaustive inquiry into, or review of, all potentially available relevant information. These statements are inherently uncertain, and you are cautioned not to rely unduly upon these statements. All information in this press release is as of the date of this press release. The information contained in any website referenced herein is not, and shall not be deemed to be, part of or incorporated into this press release.
[i] U.S. Food and Drug Administration. (2022, October 14). Final Administrative Order (OTC000027): Internal Analgesic, Antipyretic, and Antirheumatic Drug Products for Over-the-Counter Human Use.
[ii] ISIS-2 (Second International Study of Infarct Survival) Collaborative Group. Randomized trial of intravenous streptokinase, oral aspirin, both, or neither among 17,187 cases of suspected acute myocardial infarction: ISIS-2. Lancet. 1988; 2:349-60.
[iii] Rao SV, O'Donoghue ML, Ruel M, et al. 2025 ACC/AHA/ACEP/NAEMSP/SCAI Guideline for the Management of Patients with Acute Coronary Syndromes: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2025; 151:e771-e862.
Aspire Biopharma Holdings, Inc.
Contact
PCG Advisory
Kevin McGrath
+1-646-418-7002
[email protected]
SOURCE: Aspire Biopharma Holdings, Inc.
View the original press release on ACCESS Newswire
M.Thompson--AMWN