
-
 Telefonica to shed around 5,500 jobs in Spain
Telefonica to shed around 5,500 jobs in Spain
-
McCullum wants to stay as England coach despite Ashes drubbing

-
 EU slams China dairy duties as 'unjustified'
EU slams China dairy duties as 'unjustified'
-
Italy fines Apple nearly 100 mn euros over app privacy feature

-
 America's Cup switches to two-year cycle
America's Cup switches to two-year cycle
-
Jesus could start for Arsenal in League Cup, says Arteta

-
 EU to probe Czech aid for two nuclear units
EU to probe Czech aid for two nuclear units
-
Strauss says sacking Stokes and McCullum will not solve England's Ashes woes

-
 Noel takes narrow lead after Alta Badia slalom first run
Noel takes narrow lead after Alta Badia slalom first run
-
Stocks diverge as rate hopes rise, AI fears ease

-
 Man City players face Christmas weigh-in as Guardiola issues 'fatty' warning
Man City players face Christmas weigh-in as Guardiola issues 'fatty' warning
-
German Christmas markets hit by flood of fake news

-
 Liverpool fear Isak has broken leg: reports
Liverpool fear Isak has broken leg: reports
-
West Indies captain says he 'let the team down' in New Zealand Tests

-
 Thailand says Cambodia agrees to border talks after ASEAN meet
Thailand says Cambodia agrees to border talks after ASEAN meet
-
Alleged Bondi shooters conducted 'tactical' training in countryside, Australian police say

-
 Swiss court to hear landmark climate case against cement giant
Swiss court to hear landmark climate case against cement giant
-
Knicks' Brunson scores 47, Bulls edge Hawks epic

-
 Global nuclear arms control under pressure in 2026
Global nuclear arms control under pressure in 2026
-
Asian markets rally with Wall St as rate hopes rise, AI fears ease

-
 Jailed Malaysian ex-PM Najib loses bid for house arrest
Jailed Malaysian ex-PM Najib loses bid for house arrest
-
Banned film exposes Hong Kong's censorship trend, director says

-
 Duffy, Patel force West Indies collapse as NZ close in on Test series win
Duffy, Patel force West Indies collapse as NZ close in on Test series win
-
Australian state pushes tough gun laws, 'terror symbols' ban after shooting

-
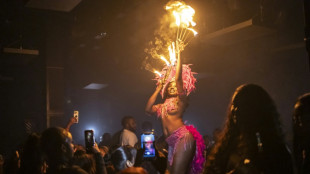 A night out on the town during Nigeria's 'Detty December'
A night out on the town during Nigeria's 'Detty December'
-
US in 'pursuit' of third oil tanker in Caribbean: official

-
 CO2 soon to be buried under North Sea oil platform
CO2 soon to be buried under North Sea oil platform
-
Steelers edge Lions as Bears, 49ers reach playoffs

-
 India's Bollywood counts costs as star fees squeeze profits
India's Bollywood counts costs as star fees squeeze profits
-
McCullum admits errors in Ashes preparations as England look to salvage pride

-
 Pets, pedis and peppermints: When the diva is a donkey
Pets, pedis and peppermints: When the diva is a donkey
-
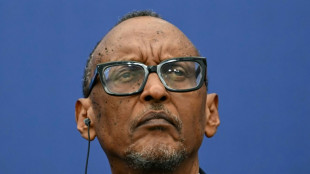
'A den of bandits': Rwanda closes thousands of evangelical churches
-
 Southeast Asia bloc meets to press Thailand, Cambodia on truce
Southeast Asia bloc meets to press Thailand, Cambodia on truce
-
As US battles China on AI, some companies choose Chinese

-
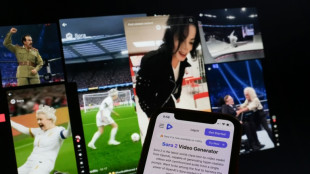 AI resurrections of dead celebrities amuse and rankle
AI resurrections of dead celebrities amuse and rankle
-
NESR Becomes First Oilfield Services Company to Commission Original Artwork Created from Recycled Produced Water
-
 SMX Strikes Joint Initiative with FinGo & Bougainville Refinery Ltd to Deliver Verifiable Identification for Trillion Dollar Gold Market
SMX Strikes Joint Initiative with FinGo & Bougainville Refinery Ltd to Deliver Verifiable Identification for Trillion Dollar Gold Market
-
Blue Gold and Trust Stamp Execute Strategic LOI to Develop Biometric, Passwordless Wallet Infrastructure for Gold-Backed Digital Assets

-
 SK tes Announces Grand Opening of New Shannon Facility, Marking a Milestone for Sustainable Technology in Ireland
SK tes Announces Grand Opening of New Shannon Facility, Marking a Milestone for Sustainable Technology in Ireland
-
FDA Officially Confirms Kava is a Food Under Federal Law

-
 Greenliant NVMe NANDrive(TM) SSDs Selected for Major Industrial, Aerospace and Mission Critical Programs
Greenliant NVMe NANDrive(TM) SSDs Selected for Major Industrial, Aerospace and Mission Critical Programs
-
World Renowned Law Firm Grant & Eisenhofer Files Class Action Lawsuit Against Canadian Banks CIBC and RBC Alleging Illegal Stock Market Manipulation of Quantum BioPharma Shares

-
 NextTrip Announces Pricing of Private Placement Financing of $3 Million
NextTrip Announces Pricing of Private Placement Financing of $3 Million
-
Namibia Critical Metals Inc. Receives Proceeds of $1,154,762 from Exercise of Warrants

-
 Shareholders Updates
Shareholders Updates
-
Applied Energetics Selected to Participate in Missile Defense Agency's Golden Dome (SHIELD) Multiple Award IDIQ Contract Vehicle

-
 Prospect Ridge Updates Diamond Drill Program at 100% Owned Camelot Copper-Gold Project in B.C.'S Cariboo Mining District
Prospect Ridge Updates Diamond Drill Program at 100% Owned Camelot Copper-Gold Project in B.C.'S Cariboo Mining District
-
The Alkaline Water Company Receives SEC Qualification of Tier 1 Regulation A Offering of Up to $10 Million

-
 Public Can Help Rid Oceans of Mines in New Freelancer Global Challenge
Public Can Help Rid Oceans of Mines in New Freelancer Global Challenge
-
Shareholders Update Report


A Drug that Could Reduce Metastatic Cancer Resurgence due to Its Anti-Inflammatory Effects in Viral Infections is in Clinical Trials
SHELTON, CONNECTICUT / ACCESS Newswire / August 18, 2025 / NanoViricides, Inc., a publicly traded company (NYSE American:NNVC) (the "Company"), and a clinical stage, leading global pioneer in the development of broad-spectrum antivirals based on host-mimetic nanomedicine technology that viruses cannot escape, reports that its broad-spectrum antiviral drug NV-387 could help reduce resurgence of metastatic cancer caused by awakening of "sleeping" cancer cells due to viral infections. NV-387 has successfully completed a Phase I clinical trial and is being advanced into Phase II clinical trials.
Recently, increase in inflammation, and particularly the cytokine IL-6, caused by viral infections has been found to be linked to an increased risk of resurgence of metastatic cancer, in a study of COVID-19 (SARS-CoV-2 infection) and breast cancer, as well as Influenza virus infections and cancer [1] . This study has further substantiated that viral infections can lead to resurgence of metastatic cancers by re-activating "sleeping" cancer cells.
"NV-387 is a remarkable antiviral drug, in that it not only attacks the virus, but also reduces inflammation, calming the human immune system so that untoward effects do not take place," said Anil R. Diwan, Ph.D., President and Chairman of the Company, adding, "We have found that NV-387 treatment reduces inflammation markers, particularly, IL-6, thereby protecting lungs. This reduction in inflammation and cyto-protective effect of NV-387 would also help minimize the risk of reawakening cancer, based on these reported studies."
Thus NV-387 treatment could have a strong impact in the treatment of cancer patients in remission who suffer from a viral infection that could lead to the cancer returning with metastasis to multiple sites in the body.
COVID-19 has become endemic globally, with generally two waves every year. The summer surge is already occurring in the USA, with approximately 4,000 hospitalizations per week in the week ending July 26 at the beginning of the surge, fueled by ever-changing variants [2] . Influenza virus is a well-known endemic virus that causes pandemics globally. RSV is an endemic virus that causes particularly severe diseases in infants and children as well as adults. Measles virus causes "immune system amnesia" and is increasing globally.
NV-387 is the only antiviral drug that has been uniquely found to be effective in the treatment of lethal lung viral infections caused by all of these viruses in animal model studies that are predictive of human clinical effectiveness.
This clearly demonstrates the unmet medical need for NV-387, a broad-spectrum antiviral that the viruses cannot escape even as new variants are created. In contrast, antibodies and vaccines readily fail with new variants arising, as is now well known.
NV-387 treatment has been found to reduce the level of IL-6 in lethal viral infections causing lung diseases in animal models. Excellent protection of lungs was found to occur in NV-387-treated animals but not in ribavirin treated animals in a lethal RSV infection study. Strong protection of lungs was found to occur in NV-387-treated animals in a lethal lung-infection of Influenza A/H3N2 study.
In this Influenza study, the approved drugs Tamiflu (oseltamivir), Rapivab (peramivib), and Xofluza (baloxavir) failed to protect the lungs of animals to any appreciable extent, indicating the superiority and clinical viability of NV-387.
The Company further investigated the extra-ordinary effect of NV-387 on protection of lungs in lethal viral infections. Reduction in inflammatory cytokines, particularly IL-6, was found to occur to an appreciable extent in NV-387 treated animals.
NanoViricides, Inc. (the "Company") (www.nanoviricides.com) is a publicly traded (NYSE-American, stock symbol NNVC) clinical stage company that is creating special purpose nanomaterials for antiviral therapy. The Company's novel nanoviricide™ class of drug candidates and the nanoviricide™ technology are based on intellectual property, technology and proprietary know-how of TheraCour Pharma, Inc. The Company has a Memorandum of Understanding with TheraCour for the development of drugs based on these technologies for all antiviral infections. The MoU does not include cancer and similar diseases that may have viral origin but require different kinds of treatments.
The Company has obtained broad, exclusive, sub-licensable, field licenses to drugs developed in several licensed fields from TheraCour Pharma, Inc. The Company's business model is based on licensing technology from TheraCour Pharma Inc. for specific application verticals of specific viruses, as established at its foundation in 2005.
Our lead drug candidate is NV-387, a broad-spectrum antiviral drug that we plan to develop as a treatment of RSV, COVID, Long COVID, Influenza, and other respiratory viral infections, as well as MPOX/Smallpox infections. Our other advanced drug candidate is NV-HHV-1 for the treatment of Shingles. The Company cannot project an exact date for filing an IND for any of its drugs because of dependence on a number of external collaborators and consultants. The Company is currently focused on advancing NV-387 into Phase II human clinical trials.
The Company is also developing drugs against a number of viral diseases including oral and genital Herpes, viral diseases of the eye including EKC and herpes keratitis, H1N1 swine flu, H5N1 bird flu, seasonal Influenza, HIV, Hepatitis C, Rabies, Dengue fever, and Ebola virus, among others. NanoViricides' platform technology and programs are based on the TheraCour® nanomedicine technology of TheraCour, which TheraCour licenses from AllExcel. NanoViricides holds a worldwide exclusive perpetual license to this technology for several drugs with specific targeting mechanisms in perpetuity for the treatment of the following human viral diseases: Human Immunodeficiency Virus (HIV/AIDS), Hepatitis B Virus (HBV), Hepatitis C Virus (HCV), Rabies, Herpes Simplex Virus (HSV-1 and HSV-2), Varicella-Zoster Virus (VZV), Influenza and Asian Bird Flu Virus, Dengue viruses, Japanese Encephalitis virus, West Nile Virus, Ebola/Marburg viruses, and certain Coronaviruses. The Company intends to obtain a license for RSV, Poxviruses, and/or Enteroviruses if the initial research is successful. As is customary, the Company must state the risk factor that the path to typical drug development of any pharmaceutical product is extremely lengthy and requires substantial capital. As with any drug development efforts by any company, there can be no assurance at this time that any of the Company's pharmaceutical candidates would show sufficient effectiveness and safety for human clinical development. Further, there can be no assurance at this time that successful results against coronavirus in our lab will lead to successful clinical trials or a successful pharmaceutical product.
This press release contains forward-looking statements that reflect the Company's current expectation regarding future events. Actual events could differ materially and substantially from those projected herein and depend on a number of factors. Certain statements in this release, and other written or oral statements made by NanoViricides, Inc. are "forward-looking statements" within the meaning of Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934. You should not place undue reliance on forward-looking statements since they involve known and unknown risks, uncertainties and other factors which are, in some cases, beyond the Company's control and which could, and likely will, materially affect actual results, levels of activity, performance or achievements. The Company assumes no obligation to publicly update or revise these forward-looking statements for any reason, or to update the reasons actual results could differ materially from those anticipated in these forward-looking statements, even if new information becomes available in the future. Important factors that could cause actual results to differ materially from the company's expectations include, but are not limited to, those factors that are disclosed under the heading "Risk Factors" and elsewhere in documents filed by the company from time to time with the United States Securities and Exchange Commission and other regulatory authorities. Although it is not possible to predict or identify all such factors, they may include the following: demonstration and proof of principle in preclinical trials that a nanoviricide is safe and effective; successful development of our product candidates; our ability to seek and obtain regulatory approvals, including with respect to the indications we are seeking; the successful commercialization of our product candidates; and market acceptance of our products.
The phrases "safety", "effectiveness" and equivalent phrases as used in this press release refer to research findings including clinical trials as the customary research usage and do not indicate evaluation of safety or effectiveness by the US FDA.
FDA refers to US Food and Drug Administration. IND application refers to "Investigational New Drug" application. cGMP refers to current Good Manufacturing Practices. CMC refers to "Chemistry, Manufacture, and Controls". CHMP refers to the Committee for Medicinal Products for Human Use, which is the European Medicines Agency's (EMA) committee responsible for human medicines. API stands for "Active Pharmaceutical Ingredient". WHO is the World Health Organization. R&D refers to Research and Development.
Contact:
NanoViricides, Inc.
[email protected]
Public Relations Contact:
[email protected]
[1] https://news.cuanschutz.edu/cancer-center/covid-19-awaken-dormant-cancer-cells . Publication: "Respiratory viral infections awaken metastatic breast cancer cells in lungs", Chia et al, J DeGregori group, https://doi.org/10.1038/s41586-025-09332-0.
[2] Centers for Disease Control and Prevention. COVID Data Tracker. Atlanta, GA: U.S. Department of Health and Human Services, CDC; 2025, August 18. https://covid.cdc.gov/covid-data-tracker.
SOURCE: NanoViricides, Inc.
View the original press release on ACCESS Newswire
P.Martin--AMWN