
-
 Telefonica to shed around 5,500 jobs in Spain
Telefonica to shed around 5,500 jobs in Spain
-
McCullum wants to stay as England coach despite Ashes drubbing

-
 EU slams China dairy duties as 'unjustified'
EU slams China dairy duties as 'unjustified'
-
Italy fines Apple nearly 100 mn euros over app privacy feature

-
 America's Cup switches to two-year cycle
America's Cup switches to two-year cycle
-
Jesus could start for Arsenal in League Cup, says Arteta

-
 EU to probe Czech aid for two nuclear units
EU to probe Czech aid for two nuclear units
-
Strauss says sacking Stokes and McCullum will not solve England's Ashes woes

-
 Noel takes narrow lead after Alta Badia slalom first run
Noel takes narrow lead after Alta Badia slalom first run
-
Stocks diverge as rate hopes rise, AI fears ease

-
 Man City players face Christmas weigh-in as Guardiola issues 'fatty' warning
Man City players face Christmas weigh-in as Guardiola issues 'fatty' warning
-
German Christmas markets hit by flood of fake news

-
 Liverpool fear Isak has broken leg: reports
Liverpool fear Isak has broken leg: reports
-
West Indies captain says he 'let the team down' in New Zealand Tests

-
 Thailand says Cambodia agrees to border talks after ASEAN meet
Thailand says Cambodia agrees to border talks after ASEAN meet
-
Alleged Bondi shooters conducted 'tactical' training in countryside, Australian police say

-
 Swiss court to hear landmark climate case against cement giant
Swiss court to hear landmark climate case against cement giant
-
Knicks' Brunson scores 47, Bulls edge Hawks epic

-
 Global nuclear arms control under pressure in 2026
Global nuclear arms control under pressure in 2026
-
Asian markets rally with Wall St as rate hopes rise, AI fears ease

-
 Jailed Malaysian ex-PM Najib loses bid for house arrest
Jailed Malaysian ex-PM Najib loses bid for house arrest
-
Banned film exposes Hong Kong's censorship trend, director says

-
 Duffy, Patel force West Indies collapse as NZ close in on Test series win
Duffy, Patel force West Indies collapse as NZ close in on Test series win
-
Australian state pushes tough gun laws, 'terror symbols' ban after shooting

-
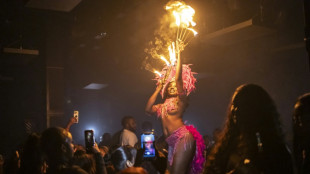 A night out on the town during Nigeria's 'Detty December'
A night out on the town during Nigeria's 'Detty December'
-
US in 'pursuit' of third oil tanker in Caribbean: official

-
 CO2 soon to be buried under North Sea oil platform
CO2 soon to be buried under North Sea oil platform
-
Steelers edge Lions as Bears, 49ers reach playoffs

-
 India's Bollywood counts costs as star fees squeeze profits
India's Bollywood counts costs as star fees squeeze profits
-
McCullum admits errors in Ashes preparations as England look to salvage pride

-
 Pets, pedis and peppermints: When the diva is a donkey
Pets, pedis and peppermints: When the diva is a donkey
-
'A den of bandits': Rwanda closes thousands of evangelical churches
-
 Southeast Asia bloc meets to press Thailand, Cambodia on truce
Southeast Asia bloc meets to press Thailand, Cambodia on truce
-
As US battles China on AI, some companies choose Chinese

-
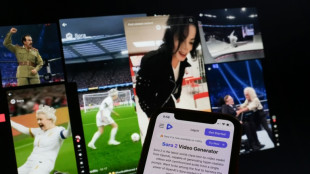 AI resurrections of dead celebrities amuse and rankle
AI resurrections of dead celebrities amuse and rankle
-
NESR Becomes First Oilfield Services Company to Commission Original Artwork Created from Recycled Produced Water
-
 SMX Strikes Joint Initiative with FinGo & Bougainville Refinery Ltd to Deliver Verifiable Identification for Trillion Dollar Gold Market
SMX Strikes Joint Initiative with FinGo & Bougainville Refinery Ltd to Deliver Verifiable Identification for Trillion Dollar Gold Market
-
Blue Gold and Trust Stamp Execute Strategic LOI to Develop Biometric, Passwordless Wallet Infrastructure for Gold-Backed Digital Assets

-
 SK tes Announces Grand Opening of New Shannon Facility, Marking a Milestone for Sustainable Technology in Ireland
SK tes Announces Grand Opening of New Shannon Facility, Marking a Milestone for Sustainable Technology in Ireland
-
FDA Officially Confirms Kava is a Food Under Federal Law

-
 Greenliant NVMe NANDrive(TM) SSDs Selected for Major Industrial, Aerospace and Mission Critical Programs
Greenliant NVMe NANDrive(TM) SSDs Selected for Major Industrial, Aerospace and Mission Critical Programs
-
World Renowned Law Firm Grant & Eisenhofer Files Class Action Lawsuit Against Canadian Banks CIBC and RBC Alleging Illegal Stock Market Manipulation of Quantum BioPharma Shares

-
 NextTrip Announces Pricing of Private Placement Financing of $3 Million
NextTrip Announces Pricing of Private Placement Financing of $3 Million
-
Namibia Critical Metals Inc. Receives Proceeds of $1,154,762 from Exercise of Warrants

-
 Shareholders Updates
Shareholders Updates
-
Applied Energetics Selected to Participate in Missile Defense Agency's Golden Dome (SHIELD) Multiple Award IDIQ Contract Vehicle

-
 Prospect Ridge Updates Diamond Drill Program at 100% Owned Camelot Copper-Gold Project in B.C.'S Cariboo Mining District
Prospect Ridge Updates Diamond Drill Program at 100% Owned Camelot Copper-Gold Project in B.C.'S Cariboo Mining District
-
The Alkaline Water Company Receives SEC Qualification of Tier 1 Regulation A Offering of Up to $10 Million

-
 Public Can Help Rid Oceans of Mines in New Freelancer Global Challenge
Public Can Help Rid Oceans of Mines in New Freelancer Global Challenge
-
Shareholders Update Report


Ensysce Biosciences' Geoff Birkett to Chair the Fierce New Product Planning Summit 2025
~ The Summit is the Premier Industry Event for Defining How New Product Planning Teams and Related Functions Make Informed, Value-Based, Data-Driven Decisions ~
~ Ensysce Biopharma Leader to Deliver Opening Remarks and Present Case Study on Launching Successful Brands ~
SAN DIEGO, CA / ACCESS Newswire / August 19, 2025 / Ensysce Biosciences, Inc. (NASDAQ:ENSC), a clinical-stage company pioneering novel solutions for severe pain with built-in abuse and overdose protection, today announced that Geoff Birkett, Chief Commercial Officer of Ensysce, will chair the Fierce New Product Planning Summit 2025, held September 8-11, 2025 at the Pennsylvania Convention Center in Philadelphia.
Mr. Birkett, recognized for his expertise in product launches and commercial strategy, will deliver the Opening Remarks, set the tone of the meeting, and present a case study on the launch process and how to maximize success.
New Product Planning Summit, Tailored Product Commercialization Strategies
The Fierce New Product Planning Summit is the premier industry event for defining how new product planning teams and related functions make informed, value-based, data-driven decisions to ensure commercial success of products in development. Life science leaders convene annually to share insights on maximizing pipeline potential through disciplined strategy and execution. Please find more information here.
Geoff Birkett, Chief Commercial Officer Ensysce
Mr. Birkett successfully brought to market several groundbreaking medicines in pain, addiction, and neuroscience. At Ensysce, he is currently spearheading preparations for the launch of the Company's first-in-class analgesic, PF614, and shaping a portfolio of innovative therapies to combat severe pain while reducing the risks of abuse and overdose.
Throughout his career, Mr. Birkett has led development and commercialization teams from early-phase programs through launch and lifecycle management. He began his career in biochemistry in Newcastle, England, before joining Eli Lilly, then Lundbeck, where he led UK sales and marketing. At ICI Pharmaceuticals and AstraZeneca (AZ), he served for 15 years, ultimately overseeing the AZ merger process in all ex-US markets and rising to Global SVP for CNS and Oncology. He holds advanced business qualifications from INSEAD and Henley Business Schools.
About Ensysce Biosciences
Ensysce Biosciences is a clinical stage company with a goal of disrupting the analgesic landscape by introducing a new class of highly novel opioids for the treatment of severe pain. Leveraging its Trypsin-Activated Abuse Protection (TAAPTM) and Multi-Pill Abuse Resistance (MPAR®) platforms, the Company is developing unique, tamper-proof treatment options for pain that minimize the risk of both drug abuse and overdose. Ensysce's products are anticipated to provide safer options to treat patients suffering from severe pain and assist in preventing deaths caused by medication abuse. Ensysce's pipeline is backed by a robust global intellectual property portfolio, offering hope to patients and providers confronting the challenges of pain management. Learn more at www.ensysce.com.
Forward-Looking Statements
Statements contained in this press release that are not purely historical may be deemed to be forward-looking statements for the purposes of the safe harbor provisions under The Private Securities Litigation Reform Act of 1995 and other federal securities laws. Without limiting the foregoing, the use of words such as "may," "intends," "can," "might," "will," "expect," "plan," "possible," "believe" and other similar expressions are intended to identify forward-looking statements. The product candidates discussed are in clinic and not approved and there can be no assurance that the clinical programs will be successful in demonstrating safety and/or efficacy, that Ensysce will not encounter problems or delays in clinical development, or that any product candidate will ever receive regulatory approval or be successfully commercialized. All forward-looking statements are based on estimates and assumptions by Ensysce's management that, although Ensysce believes to be reasonable, are inherently uncertain. All forward-looking statements are subject to risks and uncertainties that may cause actual results to differ materially from those that Ensysce expected. In addition, Ensysce's business is subject to additional risks and uncertainties, including among others, the initiation and conduct of preclinical studies and clinical trials; the timing and availability of data from preclinical studies and clinical trials; expectations for regulatory submissions and approvals; potential safety concerns related to, or efficacy of, Ensysce's product candidates; the availability or commercial potential of product candidates; the ability of Ensysce to fund its continued operations, including its planned clinical trials; the dilutive effect of stock issuances from our fundraising; and Ensysce's and its partners' ability to perform under their license, collaboration and manufacturing arrangements. These statements are also subject to a number of material risks and uncertainties that are described in Ensysce's most recent quarterly report on Form 10-Q and current reports on Form 8-K, available free of charge at the SEC's website at www.sec.gov. Any forward-looking statement speaks only as of the date on which it was made. Ensysce undertakes no obligation to publicly update or revise any forward-looking statement, whether as a result of new information, future events or otherwise, except as required under applicable law.
Ensysce Biosciences Company Contact:
Lynn Kirkpatrick, Ph.D.
Chief Executive Officer
(858) 263-4196
Ensysce Biosciences Investor Relations Contact:
Shannon Devine
MZ North America
Main: 203-741-8811
[email protected]
SOURCE: Ensysce Biosciences Inc.
View the original press release on ACCESS Newswire
M.Thompson--AMWN