
-
 America's Cup switches to two-year cycle
America's Cup switches to two-year cycle
-
Jesus could start for Arsenal in League Cup, says Arteta

-
 EU to probe Czech aid for two nuclear units
EU to probe Czech aid for two nuclear units
-
Strauss says sacking Stokes and McCullum will not solve England's Ashes woes

-
 Noel takes narrow lead after Alta Badia slalom first run
Noel takes narrow lead after Alta Badia slalom first run
-
Stocks diverge as rate hopes rise, AI fears ease

-
 Man City players face Christmas weigh-in as Guardiola issues 'fatty' warning
Man City players face Christmas weigh-in as Guardiola issues 'fatty' warning
-
German Christmas markets hit by flood of fake news

-
 Liverpool fear Isak has broken leg: reports
Liverpool fear Isak has broken leg: reports
-
West Indies captain says he 'let the team down' in New Zealand Tests

-
 Thailand says Cambodia agrees to border talks after ASEAN meet
Thailand says Cambodia agrees to border talks after ASEAN meet
-
Alleged Bondi shooters conducted 'tactical' training in countryside, Australian police say

-
 Swiss court to hear landmark climate case against cement giant
Swiss court to hear landmark climate case against cement giant
-
Knicks' Brunson scores 47, Bulls edge Hawks epic

-
 Global nuclear arms control under pressure in 2026
Global nuclear arms control under pressure in 2026
-
Asian markets rally with Wall St as rate hopes rise, AI fears ease

-
 Jailed Malaysian ex-PM Najib loses bid for house arrest
Jailed Malaysian ex-PM Najib loses bid for house arrest
-
Banned film exposes Hong Kong's censorship trend, director says

-
 Duffy, Patel force West Indies collapse as NZ close in on Test series win
Duffy, Patel force West Indies collapse as NZ close in on Test series win
-
Australian state pushes tough gun laws, 'terror symbols' ban after shooting

-
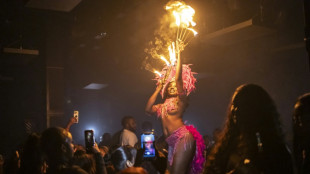 A night out on the town during Nigeria's 'Detty December'
A night out on the town during Nigeria's 'Detty December'
-
US in 'pursuit' of third oil tanker in Caribbean: official

-
 CO2 soon to be buried under North Sea oil platform
CO2 soon to be buried under North Sea oil platform
-
Steelers edge Lions as Bears, 49ers reach playoffs

-
 India's Bollywood counts costs as star fees squeeze profits
India's Bollywood counts costs as star fees squeeze profits
-
McCullum admits errors in Ashes preparations as England look to salvage pride

-
 Pets, pedis and peppermints: When the diva is a donkey
Pets, pedis and peppermints: When the diva is a donkey
-
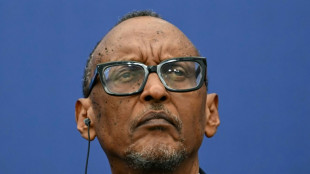
'A den of bandits': Rwanda closes thousands of evangelical churches
-
 Southeast Asia bloc meets to press Thailand, Cambodia on truce
Southeast Asia bloc meets to press Thailand, Cambodia on truce
-
As US battles China on AI, some companies choose Chinese

-
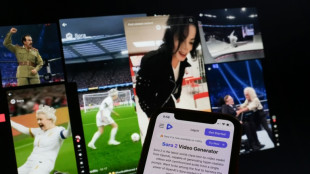 AI resurrections of dead celebrities amuse and rankle
AI resurrections of dead celebrities amuse and rankle
-
EonX Announces Update To Loan Facility

-
 Silver X Mining Announces Management Update
Silver X Mining Announces Management Update
-
Medicus Pharma Ltd. Announces Engagement With Reliant AI to Develop Artificial Intelligence (AI) Driven Clinical Data Analytics Platform

-
 Revolve Receives Generation Permit Approval for 130 MW El 24 Wind Project in Mexico
Revolve Receives Generation Permit Approval for 130 MW El 24 Wind Project in Mexico
-
NuRAN Restores Sites in Ghana and Resumes Network Deployment Activities in the Democratic Republic of the Congo

-
 1933 Industries Issues Final Reminder to 2024 Debenture Holders: December 22 Is the Deadline to Convert
1933 Industries Issues Final Reminder to 2024 Debenture Holders: December 22 Is the Deadline to Convert
-
Arrive AI to Attend CES 2026 to Engage Industry Leaders on the Future of Autonomous Delivery and AI-Driven Logistics

-
 Guanajuato Silver Receives TSXV Conditional Approval for Bolanitos Acquisition
Guanajuato Silver Receives TSXV Conditional Approval for Bolanitos Acquisition
-
Brenmiller Energy CEO Avi Brenmiller Issues Year-End Letter to Shareholders

-
 1933 Industries Achieves Positive Income in Q1 2026, Marking Third Consecutive Profitable Quarter
1933 Industries Achieves Positive Income in Q1 2026, Marking Third Consecutive Profitable Quarter
-
New Joint Initiative to Tackle Core Identity Challenge For The Global Precious Metals Industry

-
 Best Western Plus Lamplighter Inn & Conference Centre recognized with 2026 Consumer Choice Award for Hotel in London
Best Western Plus Lamplighter Inn & Conference Centre recognized with 2026 Consumer Choice Award for Hotel in London
-
CoTec Investment MagIron Acquires Reynolds Pellet Plant and Launches United States Based DR Grade Pellet and Merchant Pig Iron Strategy

-
 Saat & Saat Acquires Turkish Apparel Leader Aydinli Group, Expanding U.S. Polo Assn. Markets Across Turkey, the Middle East, Eastern Europe, and North Africa
Saat & Saat Acquires Turkish Apparel Leader Aydinli Group, Expanding U.S. Polo Assn. Markets Across Turkey, the Middle East, Eastern Europe, and North Africa
-
EON Resources Inc. Reports Management and Directors Buy an Additional 282,000 Shares of EON Class A Common Stock for a Total of 1,561,000 Shares Bought in 2025 and a Total Ownership of Over 5 million Shares

-
 Heirs Energies Agrees $750m Afreximbank Financing to Drive Long-Term Growth
Heirs Energies Agrees $750m Afreximbank Financing to Drive Long-Term Growth
-
Black Book Poll: "Governed AI" Emerges as the Deciding Factor in 2026 NHS Procurement

-
 Hemogenyx Pharmaceuticals PLC Announces Update on Admission of Shares
Hemogenyx Pharmaceuticals PLC Announces Update on Admission of Shares
-
Pantheon Resources PLC Announces Shareholder Letter and Corporate Update on Dubhe-1


MIRA Pharmaceuticals Announces Completion of Phase 1 Single Ascending Dose for Oral Ketamir-2 with No Safety Concerns, Advances to Multiple Ascending Dose Stage
Following FDA IND clearance for neuropathic pain, the Company is preparing to initiate its U.S. Phase 2a trial in Q4 2025
MIAMI, FL / ACCESS Newswire / August 19, 2025 / MIRA Pharmaceuticals, Inc. (NASDAQ:MIRA) ("MIRA" or the "Company"), a clinical-stage pharmaceutical company developing novel oral therapeutics for neurologic, neuropsychiatric, and metabolic disorders, today announced the successful completion of the Single Ascending Dose (SAD) portion of its ongoing Phase 1 clinical trial evaluating oral Ketamir-2. The study, conducted at the Hadassah Clinical Research Center in Israel under the direction of Principal Investigator Prof. Yoseph Caraco, demonstrated a favorable safety and tolerability profile, with no severe or clinically significant adverse effects observed to date.
"Completion of the SAD portion with a favorable safety and tolerability profile is an important milestone in the clinical development of Ketamir-2," said Prof. Yoseph Caraco, Principal Investigator of the Phase 1 study. "Importantly, no severe or clinically significant adverse effects have been observed to date, which supports continued progression into the Multiple Ascending Dose stage of the trial."
Phase 1 Study Overview
The study- "A Phase 1, Randomized, Double-Blind, Placebo-Controlled, Single-Centre Study of Single and Repeated Dosing of Ascending Doses, to Evaluate the Safety, Tolerability and Pharmacokinetics of Oral Ketamir-2 in Healthy Adult Subjects"-is designed to establish the safety, tolerability, and pharmacokinetics of Ketamir-2 in healthy adult volunteers.
Design: Single-center, randomized, double-blind, placebo-controlled SAD/MAD with sentinel dosing and Safety Steering Committee (SSC) reviews between cohorts.
SAD completed: Four cohorts (single doses 50 mg to 600 mg); 32 participants treated (male and female).
Safety monitoring: Extensive central nervous system (CNS) safety assessments were performed using well-validated clinical research tools:
Columbia-Suicide Severity Rating Scale (C-SSRS) - screens for suicidal ideation or behavior, supporting early detection of potential mood or psychiatric changes.
Bowdle Visual Analogue Scale (VAS) - measures possible psychedelic or dissociative effects sometimes seen with ketamine and related compounds.
Ketamine Side Effect Tool (KSET) - tracks a broad range of known ketamine-related side effects over time, including sensory, cognitive, and mood changes.
These tools provided multiple, complementary layers of safety evaluation to help detect even subtle CNS effects throughout the trial.
Status: To date, no severe or clinically significant adverse effects have been observed at any dose level in the SAD portion of the study.
While the study remains ongoing and blinded, it is worth noting that, across the pharmaceutical industry as a whole, approximately one-third of investigational drugs fail during Phase 1 due to safety concerns (Tufts Center for the Study of Drug Development). These interim observations provide encouraging context as MIRA advances Ketamir-2 through the next stage of clinical evaluation, consistent with the Company's mission to prioritize safety in every step of development.
The Company is advancing to the Multiple Ascending Dose (MAD) portion, which will evaluate three cohorts receiving daily oral doses of 150 mg, 300 mg, or 600 mg for five consecutive days in up to 24 participants.
"The emerging human safety profile complements the preclinical data we've generated, which show Ketamir-2's superior efficacy in multiple neuropathic pain models without triggering the hallmark CNS side effects of ketamine," said Dr. Itzchak Angel, Chief Scientific Advisor of MIRA. "This combination of efficacy, safety, and oral delivery positions Ketamir-2 as a promising next-generation treatment for neuropathic pain and potentially other CNS disorders"
Strategic & Commercial Potential
Ketamir-2 is a proprietary, orally bioavailable new molecular entity that selectively targets the NMDA receptor (PCP site) with low affinity and shows no significant off-target activity across a broad receptor panel. Preclinical studies have demonstrated superior performance versus ketamine, pregabalin, or gabapentin (depending on comparator and model) in gold-standard neuropathic pain models-without the dissociative effects associated with ketamine.
Neuropathic pain affects an estimated 36-51 million people in North America and represents a multi-billion-dollar market today, with long-term growth driven by diabetes prevalence, cancer survivorship, and aging-related nerve damage. MIRA intends to submit a Phase 2a clinical protocol in neuropathic pain by year-end 2025 and continue evaluating potential applications in depression, anxiety, PTSD, and localized pain, subject to ongoing results and regulatory feedback.
"We are pleased to see our Phase 1 program progress as planned," said Erez Aminov, CEO of MIRA. "Advancing into the MAD stage is an important operational step as we continue to build a rigorous safety and PK foundation for Ketamir-2 and work to deliver a differentiated, non-opioid option for patients with neuropathic pain"
About MIRA Pharmaceuticals, Inc.
MIRA Pharmaceuticals, Inc. (NASDAQ:MIRA) is a clinical-stage pharmaceutical company focused on the development and commercialization of novel therapeutics for neurologic, neuropsychiatric, and metabolic disorders. The Company's pipeline includes oral drug candidates designed to address significant unmet medical needs in areas such as neuropathic pain, inflammatory pain, obesity, addiction, anxiety, and cognitive decline.
For more information, please visit www.mirapharmaceuticals.com.
Cautionary Note Regarding Forward-Looking Statements
This press release and the statements of MIRA's management related thereto contain "forward-looking statements," which are statements other than historical facts made pursuant to the safe harbor provisions of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended. These statements may be identified by words such as "aims," "anticipates," "believes," "could," "estimates," "expects," "forecasts," "goal," "intends," "may," "plans," "possible," "potential," "seeks," "will," and variations of these words or similar expressions that are intended to identify forward-looking statements. Any statements in this press release that are not historical facts may be deemed forward-looking. Any forward-looking statements in this press release are based on MIRA's current expectations, estimates, and projections only as of the date of this release and are subject to a number of risks and uncertainties (many of which are beyond MIRA's control) that could cause actual results to differ materially and adversely from those set forth in or implied by such forward-looking statements, including related to MIRA's potential merger with SKNY Pharmaceuticals, Inc. These and other risks concerning MIRA's programs and operations are described in additional detail in the Annual Report on Form 10-K for the year ended December 31, 2024, and the Form 14A filed by MIRA on June 18, 2025, and other SEC filings, which are on file with the SEC at www.sec.gov and on MIRA's website at https://www.mirapharmaceuticals.com/investors/sec-filings. MIRA explicitly disclaims any obligation to update any forward-looking statements except to the extent required by law.
Contact:
Helga Moya
[email protected]
(786) 432-9792
SOURCE: MIRA Pharmaceuticals
View the original press release on ACCESS Newswire
J.Oliveira--AMWN
