
-
 Telefonica to shed around 5,500 jobs in Spain
Telefonica to shed around 5,500 jobs in Spain
-
McCullum wants to stay as England coach despite Ashes drubbing

-
 EU slams China dairy duties as 'unjustified'
EU slams China dairy duties as 'unjustified'
-
Italy fines Apple nearly 100 mn euros over app privacy feature

-
 America's Cup switches to two-year cycle
America's Cup switches to two-year cycle
-
Jesus could start for Arsenal in League Cup, says Arteta

-
 EU to probe Czech aid for two nuclear units
EU to probe Czech aid for two nuclear units
-
Strauss says sacking Stokes and McCullum will not solve England's Ashes woes

-
 Noel takes narrow lead after Alta Badia slalom first run
Noel takes narrow lead after Alta Badia slalom first run
-
Stocks diverge as rate hopes rise, AI fears ease

-
 Man City players face Christmas weigh-in as Guardiola issues 'fatty' warning
Man City players face Christmas weigh-in as Guardiola issues 'fatty' warning
-
German Christmas markets hit by flood of fake news

-
 Liverpool fear Isak has broken leg: reports
Liverpool fear Isak has broken leg: reports
-
West Indies captain says he 'let the team down' in New Zealand Tests

-
 Thailand says Cambodia agrees to border talks after ASEAN meet
Thailand says Cambodia agrees to border talks after ASEAN meet
-
Alleged Bondi shooters conducted 'tactical' training in countryside, Australian police say

-
 Swiss court to hear landmark climate case against cement giant
Swiss court to hear landmark climate case against cement giant
-
Knicks' Brunson scores 47, Bulls edge Hawks epic

-
 Global nuclear arms control under pressure in 2026
Global nuclear arms control under pressure in 2026
-
Asian markets rally with Wall St as rate hopes rise, AI fears ease

-
 Jailed Malaysian ex-PM Najib loses bid for house arrest
Jailed Malaysian ex-PM Najib loses bid for house arrest
-
Banned film exposes Hong Kong's censorship trend, director says

-
 Duffy, Patel force West Indies collapse as NZ close in on Test series win
Duffy, Patel force West Indies collapse as NZ close in on Test series win
-
Australian state pushes tough gun laws, 'terror symbols' ban after shooting

-
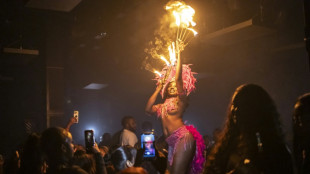 A night out on the town during Nigeria's 'Detty December'
A night out on the town during Nigeria's 'Detty December'
-
US in 'pursuit' of third oil tanker in Caribbean: official

-
 CO2 soon to be buried under North Sea oil platform
CO2 soon to be buried under North Sea oil platform
-
Steelers edge Lions as Bears, 49ers reach playoffs

-
 India's Bollywood counts costs as star fees squeeze profits
India's Bollywood counts costs as star fees squeeze profits
-
McCullum admits errors in Ashes preparations as England look to salvage pride

-
 Pets, pedis and peppermints: When the diva is a donkey
Pets, pedis and peppermints: When the diva is a donkey
-
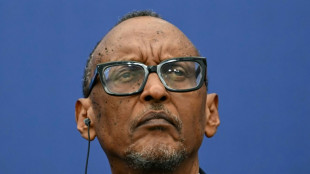
'A den of bandits': Rwanda closes thousands of evangelical churches
-
 Southeast Asia bloc meets to press Thailand, Cambodia on truce
Southeast Asia bloc meets to press Thailand, Cambodia on truce
-
As US battles China on AI, some companies choose Chinese

-
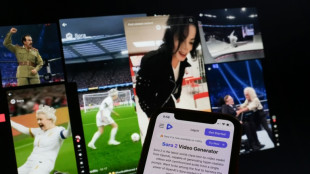 AI resurrections of dead celebrities amuse and rankle
AI resurrections of dead celebrities amuse and rankle
-
NESR Becomes First Oilfield Services Company to Commission Original Artwork Created from Recycled Produced Water
-
 SMX Strikes Joint Initiative with FinGo & Bougainville Refinery Ltd to Deliver Verifiable Identification for Trillion Dollar Gold Market
SMX Strikes Joint Initiative with FinGo & Bougainville Refinery Ltd to Deliver Verifiable Identification for Trillion Dollar Gold Market
-
Blue Gold and Trust Stamp Execute Strategic LOI to Develop Biometric, Passwordless Wallet Infrastructure for Gold-Backed Digital Assets

-
 SK tes Announces Grand Opening of New Shannon Facility, Marking a Milestone for Sustainable Technology in Ireland
SK tes Announces Grand Opening of New Shannon Facility, Marking a Milestone for Sustainable Technology in Ireland
-
FDA Officially Confirms Kava is a Food Under Federal Law

-
 Greenliant NVMe NANDrive(TM) SSDs Selected for Major Industrial, Aerospace and Mission Critical Programs
Greenliant NVMe NANDrive(TM) SSDs Selected for Major Industrial, Aerospace and Mission Critical Programs
-
World Renowned Law Firm Grant & Eisenhofer Files Class Action Lawsuit Against Canadian Banks CIBC and RBC Alleging Illegal Stock Market Manipulation of Quantum BioPharma Shares

-
 NextTrip Announces Pricing of Private Placement Financing of $3 Million
NextTrip Announces Pricing of Private Placement Financing of $3 Million
-
Namibia Critical Metals Inc. Receives Proceeds of $1,154,762 from Exercise of Warrants

-
 Shareholders Updates
Shareholders Updates
-
Applied Energetics Selected to Participate in Missile Defense Agency's Golden Dome (SHIELD) Multiple Award IDIQ Contract Vehicle

-
 Prospect Ridge Updates Diamond Drill Program at 100% Owned Camelot Copper-Gold Project in B.C.'S Cariboo Mining District
Prospect Ridge Updates Diamond Drill Program at 100% Owned Camelot Copper-Gold Project in B.C.'S Cariboo Mining District
-
The Alkaline Water Company Receives SEC Qualification of Tier 1 Regulation A Offering of Up to $10 Million

-
 Public Can Help Rid Oceans of Mines in New Freelancer Global Challenge
Public Can Help Rid Oceans of Mines in New Freelancer Global Challenge
-
Shareholders Update Report


Jaguar Health Family Company Napo Pharmaceuticals to Meet with FDA to Discuss Potential Regulatory Pathways for Crofelemer for Treatment of Ultrarare Pediatric Indication Microvillus Inclusion Disease (MVID)
As announced, initial proof-of-concept results from the ongoing investigator-initiated trial in Abu Dhabi show crofelemer reduced the required total parenteral nutrition in the first participating MVID patient by up to 27%; abstract describing results accepted for presentation at upcoming North American Society for Pediatric Gastroenterology, Hepatology and Nutrition (NASPGHAN) 2025 Annual Meeting in Chicago
SAN FRANCISCO, CA / ACCESS Newswire / August 19, 2025 / Jaguar Health, Inc.(NASDAQ:JAGX) (Jaguar) family company Napo Pharmaceuticals (Napo) today announced that it plans to meet with the U.S. Food and Drug Administration (FDA) to discuss the company's ongoing clinical development program for crofelemerfor the treatment of microvillus inclusion disease (MVID), an ultrarare pediatric disorder. Members of Napo's Scientific Advisory Board will join Napo and Jaguar representatives at the meeting.
"We're very pleased that Napo has been granted a meeting with the FDA to discuss Napo's development plans for crofelemer for MVID - a devastating pediatric disease characterized by severe malabsorption that requires life-sustaining parenteral support to meet the nutritional, fluid and electrolyte requirements of the child, and for which there no approved drug treatments," said Pravin Chaturvedi, PhD, Napo's and Jaguar's Chief Scientific Officer and Chair of the Scientific Advisory Board. "A core Napo goal for this meeting is to obtain input from the FDA on the clinical program and potential expedited regulatory pathways for this rare orphan indication."
As announced, and as presented April 26, 2025 at the Annual ELITE PED-GI Congress, the initial proof-of-concept results of the ongoing investigator-initiated trial (IIT) of a novel crofelemer powder formulation for oral solution in Abu Dhabi in the United Arab Emirates show that crofelemer reduced the required total parenteral nutrition (TPN) and supplementary intravenous fluids in the first participating MVID patient by up to 27%. An abstract describing the initial results of this trial has been accepted for presentation at the upcoming North American Society for Pediatric Gastroenterology, Hepatology and Nutrition (NASPGHAN) Annual Meeting taking place November 5-8, 2025 in Chicago.
Jaguar, through Napo, is supporting the independent proof-of-concept IIT in pediatric intestinal failure (IF) patients at Sheikh Khalifa Medical City in Abu Dhabi, and is conducting the placebo-controlled Phase 2 study of crofelemer in pediatric MVID patients with IF at sites in the U.S., European Union, and Middle East/North Africa regions under appropriate regulatory approvals in each of these geographies.
"Given the ultrarare nature of MVID, and the groundbreaking initial proof-of-concept results from the IIT in Abu Dhabi, even a small number of MVID patients showing benefit with crofelemer may allow Napo to explore pathways for expedited regulatory approval," said Lisa Conte, Jaguar's Founder and CEO.
Based on the initial findings from the IIT in Abu Dhabi, crofelemer's paradigm-shifting mechanism of action has the potential to provide a novel therapeutic option to reduce parenteral support and associated complications in MVID patients.
About the Jaguar Health Family of Companies
Jaguar Health, Inc. (Jaguar) is a commercial stage pharmaceuticals company focused on developing novel proprietary prescription medicines sustainably derived from plants from rainforest areas for people and animals with gastrointestinal distress. Jaguar family companies Napo Pharmaceuticals (Napo) and Napo Therapeutics S.p.A. focus on the development and commercialization of novel crofelemer powder for oral solution for the treatment of rare and orphan gastrointestinal disorders with intestinal failure, including MVID and short bowel syndrome.
For more information about:
Jaguar Health, visit https://jaguar.health
Napo Pharmaceuticals, visit www.napopharma.com
Napo Therapeutics, visit napotherapeutics.com
Forward-Looking Statements
Certain statements in this press release constitute "forward-looking statements." These include statements regarding Jaguar's expectation that Napo personnel will meet with the FDA to discuss Napo's development plans for crofelemer for MVID, statements regarding Jaguar's expectation that an abstract describing the results of the investigator-initiated trial in Abu Dhabi will be presented at NASPGHAN 2025, Jaguar's expectation that even a small number of MVID patients showing benefit with crofelemer may allow Napo to explore pathways for expedited regulatory approval, and Jaguar's expectation that crofelemer's paradigm-shifting mechanism of action has the potential to provide a novel therapeutic option to reduce parenteral support and associated complications in MVID patients. In some cases, you can identify forward-looking statements by terms such as "may," "will," "should," "expect," "plan," "aim," "anticipate," "could," "intend," "target," "project," "contemplate," "believe," "estimate," "predict," "potential" or "continue" or the negative of these terms or other similar expressions. The forward-looking statements in this release are only predictions. Jaguar has based these forward-looking statements largely on its current expectations and projections about future events. These forward-looking statements speak only as of the date of this release and are subject to several risks, uncertainties, and assumptions, some of which cannot be predicted or quantified and some of which are beyond Jaguar's control. Except as required by applicable law, Jaguar does not plan to publicly update or revise any forward-looking statements contained herein, whether as a result of any new information, future events, changed circumstances or otherwise.
Contact:
[email protected]
Jaguar-JAGX
SOURCE: Jaguar Health, Inc.
View the original press release on ACCESS Newswire
M.Thompson--AMWN